LAMP3 transfer via extracellular particles induces apoptosis in Sjögren's disease
- PMID: 36788255
- PMCID: PMC9929273
- DOI: 10.1038/s41598-023-28857-w
LAMP3 transfer via extracellular particles induces apoptosis in Sjögren's disease
Abstract
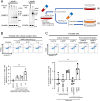
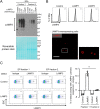
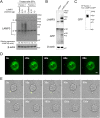
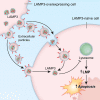
Sjögren's disease (SjD) is an autoimmune disease that affects exocrine tissues and is characterized by increased apoptosis in salivary and lacrimal glands. Although the pathogenic mechanism triggering SjD is not well understood, overexpression of lysosome-associated membrane protein 3 (LAMP3) is associated with the disease in a subset of SjD patients and the development of SjD-like phenotype in mice. In this study, histological analysis of minor salivary glands of SjD patients suggested that LAMP3-containing material is being ejected from cells. Follow-on in vitro experiments with cells exposed to extracellular particles (EPs) derived from LAMP3-overexpressing cells showed increased apoptosis. Proteomics identified LAMP3 as a major component of EPs derived from LAMP3-overexpressing cells. Live-cell imaging visualized release and uptake of LAMP3-containing EPs from LAMP3-overexpressing cells to naïve cells. Furthermore, experiments with recombinant LAMP3 protein alone or complexed with Xfect protein transfection reagent demonstrated that internalization of LAMP3 was required for apoptosis in a caspase-dependent pathway. Taken together, we identified a new role for extracellular LAMP3 in cell-to-cell communication via EPs, which provides further support for targeting LAMP3 as a therapeutic approach in SjD.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Lysosome-Associated Membrane Protein 3 Induces Lysosome-Dependent Cell Death by Impairing Autophagic Caspase 8 Degradation in the Salivary Glands of Individuals With Sjögren's Disease.Arthritis Rheumatol. 2023 Sep;75(9):1586-1598. doi: 10.1002/art.42540. Epub 2023 Jul 27. Arthritis Rheumatol. 2023. PMID: 37096570 Free PMC article.
-
LAMP3 induces apoptosis and autoantigen release in Sjögren's syndrome patients.Sci Rep. 2020 Sep 16;10(1):15169. doi: 10.1038/s41598-020-71669-5. Sci Rep. 2020. PMID: 32939030 Free PMC article.
-
Amplified Type I Interferon Response in Sjögren's Disease via Ectopic Toll-Like Receptor 7 Expression in Salivary Gland Epithelial Cells Induced by Lysosome-Associated Membrane Protein 3.Arthritis Rheumatol. 2024 Jul;76(7):1109-1119. doi: 10.1002/art.42844. Epub 2024 Jun 4. Arthritis Rheumatol. 2024. PMID: 38472139
-
Is minor salivary gland biopsy still mandatory in Sjogren's syndrome? Does seronegative Sjogren's syndrome exist?Autoimmun Rev. 2024 Jan;23(1):103425. doi: 10.1016/j.autrev.2023.103425. Epub 2023 Aug 25. Autoimmun Rev. 2024. PMID: 37634677 Review.
-
Exploring Salivary Epithelial Dysfunction in Sjögren's Disease.Int J Mol Sci. 2024 May 2;25(9):4973. doi: 10.3390/ijms25094973. Int J Mol Sci. 2024. PMID: 38732189 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

