Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19
- PMID: 36780118
- PMCID: PMC9923655
- DOI: 10.1007/s10238-023-01019-y
Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19
Abstract
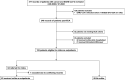
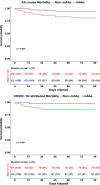
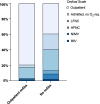
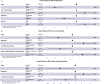
Patients with cancer have many comorbidities that increase their risk of death from Coronavirus disease 2019 (COVID-19). Anti-spike monoclonal antibodies (mAbs) reduce the risk of hospitalization or death from COVID-19 in the general population. To our knowledge, no studies have focused on the clinical efficacy of mAbs compared to no outpatient treatment exclusively among patients with solid tumors and hematologic malignancies, who are often excluded from clinical trials. We studied patients with cancer who had COVID-19 between 11.9.2020 and 7.21.2022 and received mAbs in an outpatient setting. We compared hospitalization and mortality rates to those of patients with cancer concurrently diagnosed with COVID-19, who were eligible for mAbs, but did not receive any outpatient treatment. 63 patients received mAbs and 89 no outpatient treatment. Administration of mAbs was associated with lower 90-day hospitalization (20.6% vs. 60.7%, p <0.001), all-cause (6.3% vs. 19.1%, p 0.025) and COVID-19-attributed (3.2% vs. 14.6%, p 0.019) mortality rates, and lower peak O2 requirements (ordinal Odds Ratio [OR] = 0.33, 95% Confidence Intervals [CI] = 0.20-0.53). Administration of mAbs (aHR 0.21, p <0.001), age (≥ 60 years, adjusted Hazard Ratio [aHR] 1.86, p=0.033), and metastases (aHR 0.41, p 0.007) were independently associated with hospitalization. mAb treatment remained significantly associated with all-cause (aHR 0.27, p 0.019) and COVID-19-attributed (aHR 0.19, p 0.031) mortality, after adjustment for other factors. mAb administration was associated with improved clinical outcomes among vulnerable patients with cancer and COVID-19. With no mAbs approved currently for treatment against the prevalent circulating variants, the development of new mAbs should be a research priority.
Keywords: Anti-spike monoclonal antibodies; COVID-19; Cancer; Infection; SARS-CoV-2.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
DF has received research support from Viracor, Astellas and Merck, and consultant fee from Viracor. All other authors have nothing to disclose.
Figures
Update of
-
Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19.Res Sq [Preprint]. 2023 Jan 9:rs.3.rs-2433445. doi: 10.21203/rs.3.rs-2433445/v1. Res Sq. 2023. Update in: Clin Exp Med. 2023 Oct;23(6):2739-2748. doi: 10.1007/s10238-023-01019-y PMID: 36711556 Free PMC article. Updated. Preprint.
Similar articles
-
Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19.Res Sq [Preprint]. 2023 Jan 9:rs.3.rs-2433445. doi: 10.21203/rs.3.rs-2433445/v1. Res Sq. 2023. Update in: Clin Exp Med. 2023 Oct;23(6):2739-2748. doi: 10.1007/s10238-023-01019-y PMID: 36711556 Free PMC article. Updated. Preprint.
-
Real-World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID-19 Outpatients.Chest. 2023 May;163(5):1061-1070. doi: 10.1016/j.chest.2022.10.020. Epub 2022 Oct 28. Chest. 2023. PMID: 36441040 Free PMC article.
-
Early administration of SARS-CoV-2 monoclonal antibody reduces the risk of mortality in hematologic malignancy and hematopoietic cell transplant patients with COVID-19.Transpl Infect Dis. 2023 Feb;25(1):e14006. doi: 10.1111/tid.14006. Epub 2023 Jan 27. Transpl Infect Dis. 2023. PMID: 36704987
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article. Review.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article. Review.
Cited by
-
Monovalent Omicron COVID-19 vaccine triggers superior neutralizing antibody responses against Omicron subvariants than Delta and Omicron bivalent vaccine.Hum Vaccin Immunother. 2023 Aug;19(2):2264589. doi: 10.1080/21645515.2023.2264589. Epub 2023 Oct 17. Hum Vaccin Immunother. 2023. PMID: 37846840 Free PMC article.
-
Oral antivirals for COVID-19 among patients with cancer.Support Care Cancer. 2024 Jul 9;32(8):496. doi: 10.1007/s00520-024-08714-w. Support Care Cancer. 2024. PMID: 38980433
-
Oral antivirals for COVID-19 among patients with cancer.Res Sq [Preprint]. 2024 Jan 24:rs.3.rs-3876022. doi: 10.21203/rs.3.rs-3876022/v1. Res Sq. 2024. Update in: Support Care Cancer. 2024 Jul 9;32(8):496. doi: 10.1007/s00520-024-08714-w PMID: 38343793 Free PMC article. Updated. Preprint.
References
-
- Elkrief A, Wu JT, Jani C, et al. Learning through a pandemic: the current state of knowledge on COVID-19 and cancer. Cancer Discov. 2022;12(2):303–330. doi: 10.1158/2159-8290.Cd-21-1368. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

