Mesenchymal stem cell suppresses the efficacy of CAR-T toward killing lymphoma cells by modulating the microenvironment through stanniocalcin-1
- PMID: 36779699
- PMCID: PMC10019890
- DOI: 10.7554/eLife.82934
Mesenchymal stem cell suppresses the efficacy of CAR-T toward killing lymphoma cells by modulating the microenvironment through stanniocalcin-1
Abstract
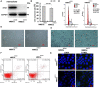
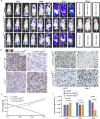
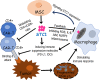
Stem cells play critical roles both in the development of cancer and therapy resistance. Although mesenchymal stem cells (MSCs) can actively migrate to tumor sites, their impact on chimeric antigen receptor modified T cell (CAR-T) immunotherapy has been little addressed. Using an in vitro cell co-culture model including lymphoma cells and macrophages, here we report that CAR-T cell-mediated cytotoxicity was significantly inhibited in the presence of MSCs. MSCs caused an increase of CD4+ T cells and Treg cells but a decrease of CD8+ T cells. In addition, MSCs stimulated the expression of indoleamine 2,3-dioxygenase and programmed cell death-ligand 1 which contributes to the immune-suppressive function of tumors. Moreover, MSCs suppressed key components of the NLRP3 inflammasome by modulating mitochondrial reactive oxygen species release. Interestingly, all these suppressive events hindering CAR-T efficacy could be abrogated if the stanniocalcin-1 (STC1) gene, which encodes the glycoprotein hormone STC-1, was knockdown in MSC. Using xenograft mice, we confirmed that CAR-T function could also be inhibited by MSC in vivo, and STC1 played a critical role. These data revealed a novel function of MSC and STC-1 in suppressing CAR-T efficacy, which should be considered in cancer therapy and may also have potential applications in controlling the toxicity arising from the excessive immune response.
Keywords: CAR-T; cancer biology; cancer therapy; human; macrophages; mesenchymal stem cells; pfeiffer cells; regenerative medicine; stanniocalcin-1; stem cells.
Plain language summary
Immunotherapy is a type of cancer treatment that helps the immune system fight cancer. For example, chimeric antigen receptor T cell (CAR-T) therapy is used to target several types of blood cancer. It works by reprogramming patients’ immune cells to target specific tumor cells. In blood cancers, CAR-T therapy works very well, but it can cause extreme responses from the patient’s immune system, which can be life threatening. In solid tumors, CAR-T therapy is much less successful because the tumors secrete molecules into the space surrounding them, which weaken the immune processes that attack cancerous cells. Stem cells are the master cells of the body. Originating in the bone marrow, they can repair and regenerate the body’s cells. Cancer stem cells play a role in resistance to CAR-T therapy, due – in part – to their ability to renew themselves, but the role of another type of stem cell, called mesenchymal stem cells, was less clear. Mesenchymal stem cells develop into tissues that line organs and blood vessels. Although it is known that mesenchymal stem cells are present in most cancers and play a role in shaping and influencing the space around tumors, their impact on CAR-T therapy has not been studied in depth. To find out more, Zhang et al. looked at the influence of a protein, called staniocalcin-1 (STC1), on CAR-T therapy, by studying cells grown in the laboratory and human tumor cells that had been implanted in mice. Zhang et al. found that mesenchymal stem cells reduce the ability of CAR-T therapy to destroy cancer cells and that they needed STC1 to do this successfully. They also increased the expression of molecules that dampen the immune system, and suppressed molecules called inflammasomes, which are an important part of the way the immune system detects disease. Moreover, reducing the amount of STC1 that mesenchymal stem cells expressed restored the effectivity of CAR-T therapy. This study increases our understanding of the way that mesenchymal stem cells affect CAR-T therapy. It has the potential to open up a new way of improving the efficiency of this treatment and of reducing the harmful side effects that it can cause.
© 2023, Zhang et al.
Conflict of interest statement
RZ, QL, SZ, HH, MZ, WM No competing interests declared
Figures


Update of
- doi: 10.1101/2022.09.21.508926
Similar articles
-
IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells.Cells. 2020 Apr 3;9(4):873. doi: 10.3390/cells9040873. Cells. 2020. PMID: 32260097 Free PMC article.
-
Mesenchymal stem cell infusion for enhancing hematopoietic recovery and addressing cytopenias in CAR-T cell therapy.Stem Cell Res Ther. 2024 Sep 27;15(1):333. doi: 10.1186/s13287-024-03941-8. Stem Cell Res Ther. 2024. PMID: 39334276 Free PMC article.
-
Survival advantage of native and engineered T cells is acquired by mitochondrial transfer from mesenchymal stem cells.J Transl Med. 2024 Sep 27;22(1):868. doi: 10.1186/s12967-024-05627-4. J Transl Med. 2024. PMID: 39334383 Free PMC article.
-
The role of MSCs and CAR-MSCs in cellular immunotherapy.Cell Commun Signal. 2023 Aug 1;21(1):187. doi: 10.1186/s12964-023-01191-4. Cell Commun Signal. 2023. PMID: 37528472 Free PMC article. Review.
-
The Role of Immunological Synapse in Predicting the Efficacy of Chimeric Antigen Receptor (CAR) Immunotherapy.Cell Commun Signal. 2020 Aug 25;18(1):134. doi: 10.1186/s12964-020-00617-7. Cell Commun Signal. 2020. PMID: 32843053 Free PMC article. Review.
Cited by
-
Novel SPEA Superantigen Peptide Agonists and Peptide Agonist-TGFαL3 Conjugate. In Vitro Study of Their Growth-Inhibitory Effects for Targeted Cancer Immunotherapy.Int J Mol Sci. 2023 Jun 22;24(13):10507. doi: 10.3390/ijms241310507. Int J Mol Sci. 2023. PMID: 37445686 Free PMC article.
-
Chimeric antigen receptor T cells in the treatment of osteosarcoma (Review).Int J Oncol. 2024 Apr;64(4):40. doi: 10.3892/ijo.2024.5628. Epub 2024 Feb 23. Int J Oncol. 2024. PMID: 38390935 Free PMC article. Review.
-
Identification and Validation of STC1 Act as a Biomarker for High-Altitude Diseases and Its Pan-Cancer Analysis.Int J Mol Sci. 2024 Aug 21;25(16):9085. doi: 10.3390/ijms25169085. Int J Mol Sci. 2024. PMID: 39201771 Free PMC article.
-
Halofuginone-guided nano-local therapy: Nano-thermosensitive hydrogels for postoperative metastatic canine mammary carcinoma with scar removal.Int J Pharm X. 2024 Mar 26;7:100241. doi: 10.1016/j.ijpx.2024.100241. eCollection 2024 Jun. Int J Pharm X. 2024. PMID: 38572023 Free PMC article.
-
Microneedle-Mediated Cell Therapy.Adv Sci (Weinh). 2024 Feb;11(8):e2304124. doi: 10.1002/advs.202304124. Epub 2023 Oct 30. Adv Sci (Weinh). 2024. PMID: 37899686 Free PMC article. Review.
References
-
- Cen S, Wang P, Xie Z, Yang R, Li J, Liu Z, Wang S, Wu X, Liu W, Li M, Tang S, Shen H, Wu Y. Autophagy enhances mesenchymal stem cell-mediated CD4+ T cell migration and differentiation through CXCL8 and TGF-β1. Stem Cell Research & Therapy. 2019;10:265. doi: 10.1186/s13287-019-1380-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

