Site-Specific Antibody Conjugation with Payloads beyond Cytotoxins
- PMID: 36770585
- PMCID: PMC9921355
- DOI: 10.3390/molecules28030917
Site-Specific Antibody Conjugation with Payloads beyond Cytotoxins
Abstract
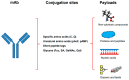
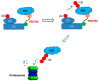
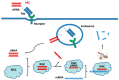
As antibody-drug conjugates have become a very important modality for cancer therapy, many site-specific conjugation approaches have been developed for generating homogenous molecules. The selective antibody coupling is achieved through antibody engineering by introducing specific amino acid or unnatural amino acid residues, peptides, and glycans. In addition to the use of synthetic cytotoxins, these novel methods have been applied for the conjugation of other payloads, including non-cytotoxic compounds, proteins/peptides, glycans, lipids, and nucleic acids. The non-cytotoxic compounds include polyethylene glycol, antibiotics, protein degraders (PROTAC and LYTAC), immunomodulating agents, enzyme inhibitors and protein ligands. Different small proteins or peptides have been selectively conjugated through unnatural amino acid using click chemistry, engineered C-terminal formylglycine for oxime or click chemistry, or specific ligation or transpeptidation with or without enzymes. Although the antibody protamine peptide fusions have been extensively used for siRNA coupling during early studies, direct conjugations through engineered cysteine or lysine residues have been demonstrated later. These site-specific antibody conjugates containing these payloads other than cytotoxic compounds can be used in proof-of-concept studies and in developing new therapeutics for unmet medical needs.
Keywords: degraders; engineering; payloads; peptides/proteins; siRNA; site-specific antibody conjugation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Site-Specifically Labeled Immunoconjugates for Molecular Imaging--Part 2: Peptide Tags and Unnatural Amino Acids.Mol Imaging Biol. 2016 Apr;18(2):153-65. doi: 10.1007/s11307-015-0920-y. Mol Imaging Biol. 2016. PMID: 26754791 Free PMC article. Review.
-
N-terminal α-amino group modification of antibodies using a site-selective click chemistry method.MAbs. 2018 Jul;10(5):712-719. doi: 10.1080/19420862.2018.1463122. MAbs. 2018. PMID: 29652547 Free PMC article.
-
Site-Specific Antibody Conjugation for ADC and Beyond.Biomedicines. 2017 Nov 9;5(4):64. doi: 10.3390/biomedicines5040064. Biomedicines. 2017. PMID: 29120405 Free PMC article. Review.
-
Impact of Drug Conjugation and Loading on Target Antigen Binding and Cytotoxicity in Cysteine Antibody-Drug Conjugates.Mol Pharm. 2021 Mar 1;18(3):889-897. doi: 10.1021/acs.molpharmaceut.0c00873. Epub 2021 Jan 20. Mol Pharm. 2021. PMID: 33470823
-
Synthesis of precision antibody conjugates using proximity-induced chemistry.Theranostics. 2021 Aug 27;11(18):9107-9117. doi: 10.7150/thno.62444. eCollection 2021. Theranostics. 2021. PMID: 34522229 Free PMC article.
Cited by
-
Recent Technological and Intellectual Property Trends in Antibody-Drug Conjugate Research.Pharmaceutics. 2024 Feb 3;16(2):221. doi: 10.3390/pharmaceutics16020221. Pharmaceutics. 2024. PMID: 38399275 Free PMC article. Review.
-
Antibody-drug conjugates in cancer therapy: mechanisms and clinical studies.MedComm (2020). 2024 Jul 28;5(8):e671. doi: 10.1002/mco2.671. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39070179 Free PMC article. Review.
-
Challenges in Permeability Assessment for Oral Drug Product Development.Pharmaceutics. 2023 Sep 28;15(10):2397. doi: 10.3390/pharmaceutics15102397. Pharmaceutics. 2023. PMID: 37896157 Free PMC article. Review.
-
Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy.Pharmaceutics. 2024 Feb 7;16(2):248. doi: 10.3390/pharmaceutics16020248. Pharmaceutics. 2024. PMID: 38399302 Free PMC article. Review.
-
Exo-Cleavable Linkers: Enhanced Stability and Therapeutic Efficacy in Antibody-Drug Conjugates.J Med Chem. 2024 Oct 24;67(20):18124-18138. doi: 10.1021/acs.jmedchem.4c01251. Epub 2024 Oct 15. J Med Chem. 2024. PMID: 39410752 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

