Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing
- PMID: 36769342
- PMCID: PMC9917831
- DOI: 10.3390/ijms24033023
Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing
Abstract
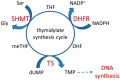
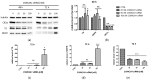
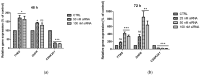
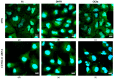
Our previous research suggests an important regulatory role of CK2-mediated phosphorylation of enzymes involved in the thymidylate biosynthesis cycle, i.e., thymidylate synthase (TS), dihydrofolate reductase (DHFR), and serine hydroxymethyltransferase (SHMT). The aim of this study was to show whether silencing of the CK2α gene affects TS and DHFR expression in A-549 cells. Additionally, we attempted to identify the endogenous kinases that phosphorylate TS and DHFR in CCRF-CEM and A-549 cells. We used immunodetection, immunofluorescence/confocal analyses, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), in-gel kinase assay, and mass spectrometry analysis. Our results demonstrate that silencing of the CK2α gene in lung adenocarcinoma cells significantly increases both TS and DHFR expression and affects their cellular distribution. Additionally, we show for the first time that both TS and DHFR are very likely phosphorylated by endogenous CK2 in two types of cancer cells, i.e., acute lymphoblastic leukaemia and lung adenocarcinoma. Moreover, our studies indicate that DHFR is phosphorylated intracellularly by CK2 to a greater extent in leukaemia cells than in lung adenocarcinoma cells. Interestingly, in-gel kinase assay results indicate that the CK2α' isoform was more active than the CK2α subunit. Our results confirm the previous studies concerning the physiological relevance of CK2-mediated phosphorylation of TS and DHFR.
Keywords: acute lymphoblastic leukaemia; dihydrofolate reductase DHFR; in-gel kinase assay; lung adenocarcinoma; protein kinase CK2; protein phosphorylation; serine hydroxymethyltransferase SHMT; thymidylate synthase TS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Inhibition of Protein Kinase CK2 Affects Thymidylate Synthesis Cycle Enzyme Level and Distribution in Human Cancer Cells.Front Mol Biosci. 2022 Feb 25;9:847829. doi: 10.3389/fmolb.2022.847829. eCollection 2022. Front Mol Biosci. 2022. PMID: 35281258 Free PMC article.
-
Simultaneous Inhibition of Protein Kinase CK2 and Dihydrofolate Reductase Results in Synergistic Effect on Acute Lymphoblastic Leukemia Cells.Anticancer Res. 2019 Jul;39(7):3531-3542. doi: 10.21873/anticanres.13499. Anticancer Res. 2019. PMID: 31262877
-
First three-dimensional structure of Toxoplasma gondii thymidylate synthase-dihydrofolate reductase: insights for catalysis, interdomain interactions, and substrate channeling.Biochemistry. 2013 Oct 15;52(41):7305-7317. doi: 10.1021/bi400576t. Epub 2013 Oct 3. Biochemistry. 2013. PMID: 24053355 Free PMC article.
-
Thymidylate synthase-dihydrofolate reductase in protozoa.Exp Parasitol. 1990 Apr;70(3):367-71. doi: 10.1016/0014-4894(90)90119-w. Exp Parasitol. 1990. PMID: 2178951 Review.
-
Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):164-73. doi: 10.1016/s0925-4439(02)00079-0. Biochim Biophys Acta. 2002. PMID: 12084458 Review.
References
-
- Mori Y., Kawamura H., Sato T., Fujita T., Nagata R., Fujihashi M., Miki K., Atomi H. Identification and enzymatic analysis of an archaeal ATP-dependent serine kinase from the hyperthermophilic archaeon Staphylothermus marinus. J. Bacteriol. 2021;203:e00025-21. doi: 10.1128/JB.00025-21. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

