Why Are Some People with Lower Urinary Tract Symptoms (LUTS) Depressed? New Evidence That Peripheral Inflammation in the Bladder Causes Central Inflammation and Mood Disorders
- PMID: 36769140
- PMCID: PMC9917564
- DOI: 10.3390/ijms24032821
Why Are Some People with Lower Urinary Tract Symptoms (LUTS) Depressed? New Evidence That Peripheral Inflammation in the Bladder Causes Central Inflammation and Mood Disorders
Abstract
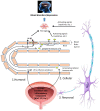
Anecdotal evidence has long suggested that patients with lower urinary tract symptoms (LUTS) develop mood disorders, such as depression and anxiety, at a higher rate than the general population and recent prospective studies have confirmed this link. Breakthroughs in our understanding of the diseases underlying LUTS have shown that many have a substantial inflammatory component and great strides have been made recently in our understanding of how this inflammation is triggered. Meanwhile, studies on mood disorders have found that many are associated with central neuroinflammation, most notably in the hippocampus. Excitingly, work on other diseases characterized by peripheral inflammation has shown that they can trigger central neuroinflammation and mood disorders. In this review, we discuss the current evidence tying LUTS to mood disorders, its possible bidirectionally, and inflammation as a common mechanism. We also review modern theories of inflammation and depression. Finally, we discuss exciting new animal studies that directly tie two bladder conditions characterized by extensive bladder inflammation (cyclophosphamide-induced hemorrhagic cystitis and bladder outlet obstruction) to neuroinflammation and depression. We conclude with a discussion of possible mechanisms by which peripheral inflammation is translated into central neuroinflammation with the resulting psychiatric concerns.
Keywords: bladder; cystitis; depression; inflammation; lower urinary tract symptoms; mood disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
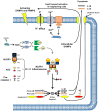
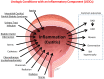
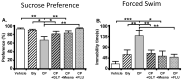
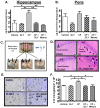
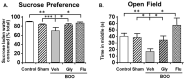
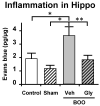
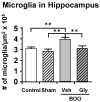
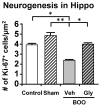
Similar articles
-
A possible mechanism underlying mood disorders associated with LUTS: Chronic bladder outlet obstruction causes NLRP3-dependent inflammation in the hippocampus and depressive behavior in rats.Neurourol Urodyn. 2020 Aug;39(6):1700-1707. doi: 10.1002/nau.24448. Epub 2020 Jun 29. Neurourol Urodyn. 2020. PMID: 32602164 Free PMC article.
-
Cyclophosphamide-induced cystitis results in NLRP3-mediated inflammation in the hippocampus and symptoms of depression in rats.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F354-F362. doi: 10.1152/ajprenal.00408.2019. Epub 2019 Dec 23. Am J Physiol Renal Physiol. 2020. PMID: 31869244 Free PMC article.
-
The role of environmental stress on lower urinary tract symptoms.Curr Opin Urol. 2017 May;27(3):268-273. doi: 10.1097/MOU.0000000000000379. Curr Opin Urol. 2017. PMID: 28376513 Review.
-
Clinical presentation, videourodynamic characteristics, and treatment outcome in men with interstitial cystitis-like lower urinary tract symptoms.Int Urol Nephrol. 2022 Sep;54(9):2157-2165. doi: 10.1007/s11255-022-03294-2. Epub 2022 Jul 8. Int Urol Nephrol. 2022. PMID: 35804206
-
Lower Urinary Tract Symptoms Including Bladder Outlet Obstruction: What's New in Diagnostics?Eur Urol Focus. 2018 Jan;4(1):14-16. doi: 10.1016/j.euf.2018.04.004. Epub 2018 Apr 14. Eur Urol Focus. 2018. PMID: 29665998 Review.
Cited by
-
Association between weight-adjusted waist index and overactive bladder syndrome among adult women in the United States: a cross-sectional study.BMC Womens Health. 2024 Sep 4;24(1):488. doi: 10.1186/s12905-024-03339-x. BMC Womens Health. 2024. PMID: 39232696 Free PMC article.
-
The Burden of Urinary Tract Infections on Quality of Life and Healthcare in Patients with Interstitial Cystitis.Healthcare (Basel). 2023 Oct 18;11(20):2761. doi: 10.3390/healthcare11202761. Healthcare (Basel). 2023. PMID: 37893834 Free PMC article.
-
Uroprotective and pain-relieving effect of dietary supplementation with micronized palmitoyl-glucosamine and hesperidin in a chronic model of cyclophosphamide-induced cystitis.Front Vet Sci. 2024 Jan 4;10:1327102. doi: 10.3389/fvets.2023.1327102. eCollection 2023. Front Vet Sci. 2024. PMID: 38249555 Free PMC article.
-
Mitigating Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Ameliorating Sexual Function and Psychological Well-Being in Older Men.Am J Mens Health. 2023 Nov-Dec;17(6):15579883231205521. doi: 10.1177/15579883231205521. Am J Mens Health. 2023. PMID: 38093710 Free PMC article.
-
Specialized pro-resolution mediators in the bladder: effects of resolvin E1 on diabetic bladder dysfunction in the type 1 diabetic male Akita mouse model.BMC Urol. 2024 Jun 21;24(1):130. doi: 10.1186/s12894-024-01519-3. BMC Urol. 2024. PMID: 38907230 Free PMC article.
References
-
- Coyne K.S., Wein A.J., Tubaro A., Sexton C.C., Thompson C.L., Kopp Z.S., Aiyer L.P. The burden of lower urinary tract symptoms: Evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103:4–11. doi: 10.1111/j.1464-410X.2009.08371.x. - DOI - PubMed
-
- Lim Y.-M., Lee S.R., Choi E.J., Jeong K., Chung H.W. Urinary incontinence is strongly associated with depression in middle-aged and older Korean women: Data from the Korean longitudinal study of ageing. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018;220:69–73. doi: 10.1016/j.ejogrb.2017.11.017. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

