Expression of CD22 in Triple-Negative Breast Cancer: A Novel Prognostic Biomarker and Potential Target for CAR Therapy
- PMID: 36768478
- PMCID: PMC9917013
- DOI: 10.3390/ijms24032152
Expression of CD22 in Triple-Negative Breast Cancer: A Novel Prognostic Biomarker and Potential Target for CAR Therapy
Abstract
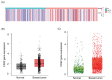
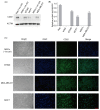
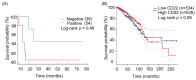
Triple-negative breast cancer (TNBC) accounts for 15-20% of all breast cancer cases. Due to the lack of expression of well-known molecular targets [estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2)], there is a need for more alternative treatment approaches in TNBC. Chimeric antigen receptor (CAR)-T cell-based immunotherapy treatment is one of the latest treatment technologies with outstanding therapeutic advances in the past decade, especially in the treatment of hematologic malignancies, but the therapeutic effects of CAR-T cells against solid tumors have not yet shown significant clinical benefits. Identification of highly specific CAR-T targets in solid tumors is also crucial for its successful treatment. CD22 is reported to be a multifunctional receptor that is mainly expressed on the surface of mature B-cells (lymphocytes) and is also highly expressed in most B-cell malignancies. This study aimed to investigate the expression of CD22 in TNBC. Bioinformatic analysis was performed to evaluate the expression of CD22 in breast carcinoma and normal tissues. RNA-seq data of normal and breast carcinoma patients were downloaded from The Cancer Genome Atlas (TCGA), and differential gene expression was performed using R language. Additionally, online bioinformatics web tools (GEPIA and TNM plot) were used to evaluate the expression of CD22 in breast carcinoma and normal tissues. Western blot (WB) analysis and immunofluorescence (IF) were performed to characterize the expression of CD22 in TNBC cell lines. Immunohistochemical (IHC) staining was performed on tumor specimens from 97 TNBC patients for CD22 expression. Moreover, statistical analysis was performed to analyze the association of clinical pathological parameters with CD22 expression. Correlation analysis between overall survival data of TNBC patients and CD22 expression was also performed. Differential gene expression analysis of TCGA data revealed that CD22 is among the upregulated differentially expressed genes (DEGs) with high expression in breast cancer, as compared to normal breast tissues. WB and IF analysis revealed high expression of CD22 in TNBC cell lines. IHC results also showed that approximately 62.89% (61/97) of TNBC specimens were stained positive for CD22. Cell membrane expression of CD22 was evident in 23.71% (23/97) of TNBC specimens, and 39.18% (38/97) of TNBC specimens showed cytoplasmic/membrane expression, while 37.11% (36/97) specimens were negative for CD22. Furthermore, significant associations were found between the size of tumors in TNBC patients and CD22 expression, which unveils its potential as a prognostic biomarker. No significant correlation was found between the overall survival of TNBC patients and CD22 expression. In conclusion, we demonstrated for the first time that CD22 is highly expressed in TNBC. Based on our findings, we anticipated that CD22 could be used as a prognostic biomarker in TNBC, and it might be a potential CAR-T target in TNBC for whom few therapeutic options exist. However, more large-scale studies and clinical trials will ensure its potential usefulness as a CAR-T target in TNBC.
Keywords: CAR-T; CD22; biomarkers; immunohistochemistry; triple negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
CD22 is a potential target of CAR-NK cell therapy for esophageal squamous cell carcinoma.J Transl Med. 2023 Oct 10;21(1):710. doi: 10.1186/s12967-023-04409-8. J Transl Med. 2023. PMID: 37817249 Free PMC article.
-
Efficacy and safety of CD22 chimeric antigen receptor (CAR) T cell therapy in patients with B cell malignancies: a protocol for a systematic review and meta-analysis.Syst Rev. 2021 Jan 21;10(1):35. doi: 10.1186/s13643-021-01588-7. Syst Rev. 2021. PMID: 33478595 Free PMC article.
-
Emerging CAR-T Cell Therapy for the Treatment of Triple-Negative Breast Cancer.Mol Cancer Ther. 2020 Dec;19(12):2409-2421. doi: 10.1158/1535-7163.MCT-20-0385. Epub 2020 Oct 21. Mol Cancer Ther. 2020. PMID: 33087511 Review.
-
CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil.Front Immunol. 2022 Nov 22;13:1018786. doi: 10.3389/fimmu.2022.1018786. eCollection 2022. Front Immunol. 2022. PMID: 36483567 Free PMC article. Review.
-
CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth.Front Immunol. 2019 May 24;10:1149. doi: 10.3389/fimmu.2019.01149. eCollection 2019. Front Immunol. 2019. PMID: 31178870 Free PMC article.
Cited by
-
Enhancing AI Research for Breast Cancer: A Comprehensive Review of Tumor-Infiltrating Lymphocyte Datasets.J Imaging Inform Med. 2024 Dec;37(6):2996-3008. doi: 10.1007/s10278-024-01043-8. Epub 2024 May 28. J Imaging Inform Med. 2024. PMID: 38806950 Free PMC article. Review.
-
Recent Advances in Breast Cancer Research.Int J Mol Sci. 2023 Jul 26;24(15):11990. doi: 10.3390/ijms241511990. Int J Mol Sci. 2023. PMID: 37569366 Free PMC article.
-
Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation.Nutrients. 2023 Oct 25;15(21):4512. doi: 10.3390/nu15214512. Nutrients. 2023. PMID: 37960165 Free PMC article.
-
CD22 is a potential target of CAR-NK cell therapy for esophageal squamous cell carcinoma.J Transl Med. 2023 Oct 10;21(1):710. doi: 10.1186/s12967-023-04409-8. J Transl Med. 2023. PMID: 37817249 Free PMC article.
-
Subtype-WGME enables whole-genome-wide multi-omics cancer subtyping.Cell Rep Methods. 2024 Jun 17;4(6):100781. doi: 10.1016/j.crmeth.2024.100781. Epub 2024 May 17. Cell Rep Methods. 2024. PMID: 38761803 Free PMC article.
References
-
- Wormann B. Breast cancer: Basics, screening, diagnostics and treatment. Med. Monatsschr. Pharm. 2017;40:55–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

