An Update of G-Protein-Coupled Receptor Signaling and Its Deregulation in Gastric Carcinogenesis
- PMID: 36765694
- PMCID: PMC9913146
- DOI: 10.3390/cancers15030736
An Update of G-Protein-Coupled Receptor Signaling and Its Deregulation in Gastric Carcinogenesis
Abstract
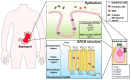
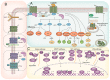
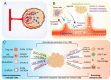
G-protein-coupled receptors (GPCRs) belong to a cell surface receptor superfamily responding to a wide range of external signals. The binding of extracellular ligands to GPCRs activates a heterotrimeric G protein and triggers the production of numerous secondary messengers, which transduce the extracellular signals into cellular responses. GPCR signaling is crucial and imperative for maintaining normal tissue homeostasis. High-throughput sequencing analyses revealed the occurrence of the genetic aberrations of GPCRs and G proteins in multiple malignancies. The altered GPCRs/G proteins serve as valuable biomarkers for early diagnosis, prognostic prediction, and pharmacological targets. Furthermore, the dysregulation of GPCR signaling contributes to tumor initiation and development. In this review, we have summarized the research progress of GPCRs and highlighted their mechanisms in gastric cancer (GC). The aberrant activation of GPCRs promotes GC cell proliferation and metastasis, remodels the tumor microenvironment, and boosts immune escape. Through deep investigation, novel therapeutic strategies for targeting GPCR activation have been developed, and the final aim is to eliminate GPCR-driven gastric carcinogenesis.
Keywords: G protein; G-protein-coupled receptor; gastric cancer; targeted therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The Ancient Link between G-Protein-Coupled Receptors and C-Terminal Phospholipid Kinase Domains.mBio. 2018 Jan 23;9(1):e02119-17. doi: 10.1128/mBio.02119-17. mBio. 2018. PMID: 29362235 Free PMC article.
-
Signaling through G protein coupled receptors.Plant Signal Behav. 2009 Oct;4(10):942-7. doi: 10.4161/psb.4.10.9530. Epub 2009 Oct 14. Plant Signal Behav. 2009. PMID: 19826234 Free PMC article. Review.
-
Targeting Gi/o protein-coupled receptor signaling blocks HER2-induced breast cancer development and enhances HER2-targeted therapy.JCI Insight. 2021 Sep 22;6(18):e150532. doi: 10.1172/jci.insight.150532. JCI Insight. 2021. PMID: 34343132 Free PMC article.
-
Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling.Pharmacol Rev. 2001 Mar;53(1):1-24. Pharmacol Rev. 2001. PMID: 11171937 Review.
-
G Protein-Coupled Receptor Signaling in Stem Cells and Cancer.Int J Mol Sci. 2016 May 11;17(5):707. doi: 10.3390/ijms17050707. Int J Mol Sci. 2016. PMID: 27187360 Free PMC article. Review.
Cited by
-
Immune-related long noncoding RNA zinc finger protein 710-AS1-201 promotes the metastasis and invasion of gastric cancer cells.World J Gastrointest Oncol. 2024 Feb 15;16(2):458-474. doi: 10.4251/wjgo.v16.i2.458. World J Gastrointest Oncol. 2024. PMID: 38425400 Free PMC article.
-
The two coin sides of bacterial extracellular membrane nanovesicles: atherosclerosis trigger or remedy.Discov Nano. 2024 Nov 12;19(1):179. doi: 10.1186/s11671-024-04149-8. Discov Nano. 2024. PMID: 39532781 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

