La2CoO4+ δ perovskite-mediated peroxymonosulfate activation for the efficient degradation of bisphenol A
- PMID: 36756419
- PMCID: PMC9854630
- DOI: 10.1039/d2ra07640c
La2CoO4+ δ perovskite-mediated peroxymonosulfate activation for the efficient degradation of bisphenol A
Abstract
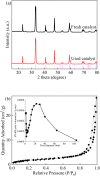
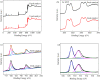
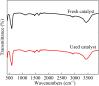
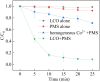
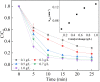
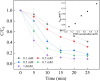
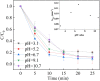
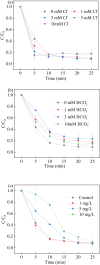
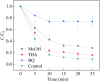
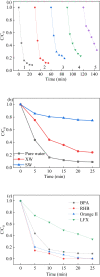
Sulfate radical-based technology has been considered as an efficient technology to remove pharmaceuticals and personal care products (PPCPs) with heterogeneous metal-mediated catalysts for the activation of peroxymonosulfate (PMS). In this study, La2CoO4+δ perovskite with Ruddlesden-Popper type structure was synthesised by the sol-gel method, which was employed in PMS activation. Different characteriazation technologies were applied for the characterization of La2CoO4+δ , such as SEM-EDX, XRD, and XPS technologies. A common organic compound, bisphenol A (BPA), is used as a target contaminant, and the effect impactors were fully investigated and explained. The results showed that when the dosage of La2CoO4+δ was 0.5 g L-1 and the concentration of PMS was 1.0 mM in neutral pH solution, about 91.1% degradation efficiency was achieved within 25 minutes. Quenching experiments were introduced in the system to verify the catalytic mechanism of PMS for the BPA degradation, proving the existence of superoxide, hydroxyl radicals and sulfate radicals, which are responsible for the catalytic degradation of BPA. Moreover, the reusability and stability of the catalyst were also conducted which showed good stability during the reaction. This work would improve the applications of A2BO4-type perovskites for activating PMS to degrade BPA.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Enhanced degradation of atrazine by nanoscale LaFe1-xCuxO3-δ perovskite activated peroxymonosulfate: Performance and mechanism.Sci Total Environ. 2019 Jul 10;673:565-575. doi: 10.1016/j.scitotenv.2019.04.098. Epub 2019 Apr 10. Sci Total Environ. 2019. PMID: 30999097
-
Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A.J Hazard Mater. 2016 Dec 15;320:150-159. doi: 10.1016/j.jhazmat.2016.08.021. Epub 2016 Aug 8. J Hazard Mater. 2016. PMID: 27544727
-
Sono-photo-assisted heterogeneous activation of peroxymonosulfate by Fe/CMK-3 catalyst for the degradation of bisphenol A, optimization with response surface methodology.Water Environ Res. 2020 Feb;92(2):189-201. doi: 10.1002/wer.1181. Epub 2019 Oct 6. Water Environ Res. 2020. PMID: 31295751
-
Degradation of atrazine in aqueous solution through peroxymonosulfate activated by Co-modified nano-titanium dioxide.Water Environ Res. 2020 Sep;92(9):1363-1375. doi: 10.1002/wer.1324. Epub 2020 May 1. Water Environ Res. 2020. PMID: 32159886
-
Efficient peroxymonosulfate activation by biochar-based nanohybrids for the degradation of pharmaceutical and personal care products in aquatic environments.Chemosphere. 2023 Jan;311(Pt 1):137084. doi: 10.1016/j.chemosphere.2022.137084. Epub 2022 Nov 2. Chemosphere. 2023. PMID: 36334754 Review.
Cited by
-
Biosynthesis of JC-La2CoO4 magnetic nanoparticles explored in catalytic and SMMs properties.Sci Rep. 2023 Dec 13;13(1):22122. doi: 10.1038/s41598-023-47852-9. Sci Rep. 2023. PMID: 38092788 Free PMC article.
References
-
- Huang L. Huang X. Yan J. Liu Y. Jiang H. Zhang H. Tang J. Liu Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2023;442:130024. - PubMed
-
- Ewis D. Ba-Abbad M. M. Benamor A. El-Naas M. H. Adsorption of organic water pollutants by clays and clay minerals composites: A comprehensive review. Appl. Clay Sci. 2022;229:106686.
-
- Shi C. Wang X. Zhou S. Zuo X. Wang C. Mechanism, application, influencing factors and environmental benefit assessment of steel slag in removing pollutants from water: A review. J. Water Process. Eng. 2022;47:102666.
-
- Wang W. Zhang H. Chen Y. Shi H. Efficient Degradation of Tetracycline via Coupling of Photocatalysis and Photo-Fenton Processes over a 2D/2D α-Fe2O3/g-C3N4 S-Scheme Heterojunction Catalyst. Acta Phys.-Chim. Sin. 2022;38:2201008.
-
- Kumar M. Sridharan S. Sawarkar A. D. Shakeel A. Anerao P. Mannina G. Sharma P. Pandey A. Current research trends on emerging contaminants pharmaceutical and personal care products (PPCPs): A comprehensive review. Sci. Total Environ. 2023;859:160031. - PubMed
LinkOut - more resources
Full Text Sources

