Persistent T cell-mediated immune responses against Omicron variants after the third COVID-19 mRNA vaccine dose
- PMID: 36756112
- PMCID: PMC9899862
- DOI: 10.3389/fimmu.2023.1099246
Persistent T cell-mediated immune responses against Omicron variants after the third COVID-19 mRNA vaccine dose
Abstract
Introduction: The prime-boost COVID-19 mRNA vaccination strategy has proven to be effective against severe COVID-19 disease and death. However, concerns have been raised due to decreasing neutralizing antibody levels after COVID-19 vaccination and due to the emergence of new immuno-evasive SARS-CoV-2 variants that may require additional booster vaccinations.
Methods: In this study, we analyzed the humoral and cell-mediated immune responses against the Omicron BA.1 and BA.2 subvariants in Finnish healthcare workers (HCWs) vaccinated with three doses of COVID-19 mRNA vaccines. We used enzyme immunoassay and microneutralization test to analyze the levels of SARS-CoV-2 specific IgG antibodies in the sera of the vaccinees and the in vitro neutralization capacity of the sera. Activation induced marker assay together with flow cytometry and extracellular cytokine analysis was used to determine responses in SARS-CoV-2 spike protein stimulated PBMCs.
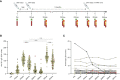
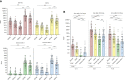
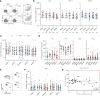
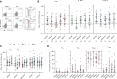
Results: Here we show that within the HCWs, the third mRNA vaccine dose recalls both humoral and T cell-mediated immune responses and induces high levels of neutralizing antibodies against Omicron BA.1 and BA.2 variants. Three weeks after the third vaccine dose, SARS-CoV-2 wild type spike protein-specific CD4+ and CD8+ T cells are observed in 82% and 71% of HCWs, respectively, and the T cells cross-recognize both Omicron BA.1 and BA.2 spike peptides. Although the levels of neutralizing antibodies against Omicron BA.1 and BA.2 decline 2.5 to 3.8-fold three months after the third dose, memory CD4+ T cell responses are maintained for at least eight months post the second dose and three months post the third vaccine dose.
Discussion: We show that after the administration of the third mRNA vaccine dose the levels of both humoral and cell-mediated immune responses are effectively activated, and the levels of the spike-specific antibodies are further elevated compared to the levels after the second vaccine dose. Even though at three months after the third vaccine dose antibody levels in sera decrease at a similar rate as after the second vaccine dose, the levels of spike-specific CD4+ and CD8+ T cells remain relatively stable. Additionally, the T cells retain efficiency in cross-recognizing spike protein peptide pools derived from Omicron BA.1 and BA.2 subvariants. Altogether our results suggest durable cellmediated immunity and protection against SARS-CoV-2.
Keywords: COVID-19; T cell responses; booster vaccine; mRNA vaccines; omicron; third vaccine dose.
Copyright © 2023 Belik, Liedes, Vara, Haveri, Pöysti, Kolehmainen, Maljanen, Huttunen, Reinholm, Lundberg, Skön, Österlund, Melin, Hänninen, Hurme, Ivaska, Tähtinen, Lempainen, Kakkola, Jalkanen and Julkunen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Immunogenicity of mRNA vs. BBV152 vaccine boosters against Omicron subvariants: Final results from Phase B of the PRIBIVAC study, a randomized clinical trial.Vaccine. 2024 Nov 14;42(25):126275. doi: 10.1016/j.vaccine.2024.126275. Epub 2024 Sep 5. Vaccine. 2024. PMID: 39241318 Clinical Trial.
-
Vaccination and Omicron BA.1/BA.2 Convalescence Enhance Systemic but Not Mucosal Immunity against BA.4/5.Microbiol Spectr. 2023 Jun 15;11(3):e0516322. doi: 10.1128/spectrum.05163-22. Epub 2023 Apr 26. Microbiol Spectr. 2023. PMID: 37098903 Free PMC article.
-
MVA-based vaccine candidates expressing SARS-CoV-2 prefusion-stabilized spike proteins of the Wuhan, Beta or Omicron BA.1 variants protect transgenic K18-hACE2 mice against Omicron infection and elicit robust and broad specific humoral and cellular immune responses.Front Immunol. 2024 Aug 29;15:1420304. doi: 10.3389/fimmu.2024.1420304. eCollection 2024. Front Immunol. 2024. PMID: 39267752 Free PMC article.
-
The humoral and cellular immune evasion of SARS-CoV-2 Omicron and sub-lineages.Virol Sin. 2022 Dec;37(6):786-795. doi: 10.1016/j.virs.2022.11.007. Epub 2022 Nov 23. Virol Sin. 2022. PMID: 36427646 Free PMC article. Review.
-
Clinical virology and effect of Covid-19 vaccination and monoclonal antibodies against highly infectious SARS- CoV-2 omicron sub variant BF.7 (BA.5.2.1.7): A systematic review.Virology. 2023 Jul;584:38-43. doi: 10.1016/j.virol.2023.04.007. Epub 2023 May 19. Virology. 2023. PMID: 37229914 Free PMC article. Review.
Cited by
-
Estimate the number of lives saved by a SARS-CoV-2 vaccination campaign in six states in the United States with a simple model.IJID Reg. 2024 Jun 15;12:100390. doi: 10.1016/j.ijregi.2024.100390. eCollection 2024 Sep. IJID Reg. 2024. PMID: 39041059 Free PMC article.
-
T cell immunity following COVID-19 vaccination in adult patients with primary antibody deficiency - a 22-month follow-up.Front Immunol. 2023 May 9;14:1146500. doi: 10.3389/fimmu.2023.1146500. eCollection 2023. Front Immunol. 2023. PMID: 37234151 Free PMC article.
-
Neutralizing antibodies after the third COVID-19 vaccination in healthcare workers with or without breakthrough infection.Commun Med (Lond). 2024 Feb 23;4(1):28. doi: 10.1038/s43856-024-00457-3. Commun Med (Lond). 2024. PMID: 38396065 Free PMC article.
-
Safety and Efficacy of Inactivated SARS-CoV-2 Vaccine in Patients with Rheumatic Diseases and Serum Antibody Changes Post-Omicron Variant Infection.Rheumatol Ther. 2024 Feb;11(1):191-200. doi: 10.1007/s40744-023-00630-5. Epub 2024 Jan 4. Rheumatol Ther. 2024. PMID: 38175331 Free PMC article.
-
Causes and Consequences of Coronavirus Spike Protein Variability.Viruses. 2024 Jan 25;16(2):177. doi: 10.3390/v16020177. Viruses. 2024. PMID: 38399953 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

