The APOBEC3B cytidine deaminase is an adenovirus restriction factor
- PMID: 36745676
- PMCID: PMC9934312
- DOI: 10.1371/journal.ppat.1011156
The APOBEC3B cytidine deaminase is an adenovirus restriction factor
Abstract
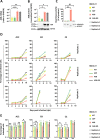
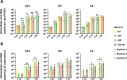
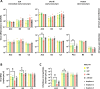
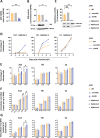
Human adenoviruses (HAdVs) are a large family of DNA viruses counting more than a hundred strains divided into seven species (A to G). HAdVs induce respiratory tract infections, gastroenteritis and conjunctivitis. APOBEC3B is a cytidine deaminase that restricts several DNA viruses. APOBEC3B is also implicated in numerous cancers where it is responsible for the introduction of clustered mutations into the cellular genome. In this study, we demonstrate that APOBEC3B is an adenovirus restriction factor acting through a deaminase-dependent mechanism. APOBEC3B introduces C-to-T clustered mutations into the adenovirus genome. APOBEC3B reduces the propagation of adenoviruses by limiting viral genome replication, progression to late phase, and production of infectious virions. APOBEC3B restriction efficiency varies between adenoviral strains, the A12 strain being more sensitive to APOBEC3B than the B3 or C2 strains. In A12-infected cells, APOBEC3B clusters in the viral replication centers. Importantly, we show that adenovirus infection leads to a reduction of the quantity and/or enzymatic activity of the APOBEC3B protein depending on the strains. The A12 strain seems less able to resist APOBEC3B than the B3 or C2 strains, a characteristic which could explain the strong depletion of the APOBEC3-targeted motifs in the A12 genome. These findings suggest that adenoviruses evolved different mechanisms to antagonize APOBEC3B. Elucidating these mechanisms could benefit the design of cancer treatments. This study also identifies adenoviruses as triggers of the APOBEC3B-mediated innate response. The involvement of certain adenoviral strains in the genesis of the APOBEC3 mutational signature observed in tumors deserves further study.
Copyright: © 2023 Lejeune et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Infection of Bronchial Epithelial Cells by the Human Adenoviruses A12, B3, and C2 Differently Regulates the Innate Antiviral Effector APOBEC3B.J Virol. 2021 Jun 10;95(13):e0241320. doi: 10.1128/JVI.02413-20. Epub 2021 Jun 10. J Virol. 2021. PMID: 33853956 Free PMC article.
-
Functional Upregulation of the DNA Cytosine Deaminase APOBEC3B by Polyomaviruses.J Virol. 2016 Jun 24;90(14):6379-6386. doi: 10.1128/JVI.00771-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147740 Free PMC article.
-
Ancestral APOBEC3B Nuclear Localization Is Maintained in Humans and Apes and Altered in Most Other Old World Primate Species.mSphere. 2022 Dec 21;7(6):e0045122. doi: 10.1128/msphere.00451-22. Epub 2022 Nov 14. mSphere. 2022. PMID: 36374108 Free PMC article.
-
Roles of APOBEC3A and APOBEC3B in Human Papillomavirus Infection and Disease Progression.Viruses. 2017 Aug 21;9(8):233. doi: 10.3390/v9080233. Viruses. 2017. PMID: 28825669 Free PMC article. Review.
-
APOBEC3B: pathological consequences of an innate immune DNA mutator.Biomed J. 2015 Mar-Apr;38(2):102-10. doi: 10.4103/2319-4170.148904. Biomed J. 2015. PMID: 25566802 Review.
Cited by
-
Differentiation signals induce APOBEC3A expression via GRHL3 in squamous epithelia and squamous cell carcinoma.EMBO J. 2025 Jan;44(1):1-29. doi: 10.1038/s44318-024-00298-9. Epub 2024 Nov 15. EMBO J. 2025. PMID: 39548236 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

