Nucleolin mediates SARS-CoV-2 replication and viral-induced apoptosis of host cells
- PMID: 36740097
- PMCID: PMC9896859
- DOI: 10.1016/j.antiviral.2023.105550
Nucleolin mediates SARS-CoV-2 replication and viral-induced apoptosis of host cells
Abstract
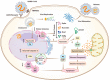
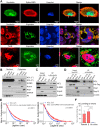
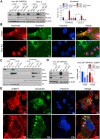
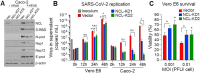
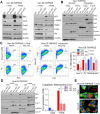
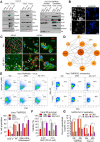
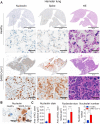
Host-oriented antiviral therapeutics are promising treatment options to combat COVID-19 and its emerging variants. However, relatively little is known about the cellular proteins hijacked by SARS-CoV-2 for its replication. Here we show that SARS-CoV-2 induces expression and cytoplasmic translocation of the nucleolar protein, nucleolin (NCL). NCL interacts with SARS-CoV-2 viral proteins and co-localizes with N-protein in the nucleolus and in stress granules. Knockdown of NCL decreases the stress granule component G3BP1, viral replication and improved survival of infected host cells. NCL mediates viral-induced apoptosis and stress response via p53. SARS-CoV-2 increases NCL expression and nucleolar size and number in lungs of infected hamsters. Inhibition of NCL with the aptamer AS-1411 decreases viral replication and apoptosis of infected cells. These results suggest nucleolin as a suitable target for anti-COVID therapies.
Keywords: Aptamer; COVID; Nucleolin; Nucleoprotein; SARS-CoV-2.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Japanese encephalitis virus NS5 protein interacts with nucleolin to enhance the virus replication.J Virol. 2024 Aug 20;98(8):e0085824. doi: 10.1128/jvi.00858-24. Epub 2024 Jul 30. J Virol. 2024. PMID: 39078257
-
Proteomic analysis identifies the RNA helicase DDX3X as a host target against SARS-CoV-2 infection.Antiviral Res. 2021 Jun;190:105064. doi: 10.1016/j.antiviral.2021.105064. Epub 2021 Mar 26. Antiviral Res. 2021. PMID: 33781803 Free PMC article.
-
SARS-CoV-2 N Protein Antagonizes Stress Granule Assembly and IFN Production by Interacting with G3BPs to Facilitate Viral Replication.J Virol. 2022 Jun 22;96(12):e0041222. doi: 10.1128/jvi.00412-22. Epub 2022 Jun 2. J Virol. 2022. PMID: 35652658 Free PMC article.
-
The roles of G3BP1 in human diseases (review).Gene. 2022 May 5;821:146294. doi: 10.1016/j.gene.2022.146294. Epub 2022 Feb 14. Gene. 2022. PMID: 35176431 Review.
-
Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1.Viruses. 2023 Feb 6;15(2):449. doi: 10.3390/v15020449. Viruses. 2023. PMID: 36851663 Free PMC article. Review.
Cited by
-
Integrated analysis of RNA-seq datasets reveals novel targets and regulators of COVID-19 severity.Life Sci Alliance. 2024 Jan 23;7(4):e202302358. doi: 10.26508/lsa.202302358. Print 2024 Apr. Life Sci Alliance. 2024. PMID: 38262689 Free PMC article.
-
Insights into the Cellular Localization and Functional Properties of TSPYL5 Protein.Int J Mol Sci. 2023 Dec 19;25(1):39. doi: 10.3390/ijms25010039. Int J Mol Sci. 2023. PMID: 38203210 Free PMC article.
-
Japanese encephalitis virus NS5 protein interacts with nucleolin to enhance the virus replication.J Virol. 2024 Aug 20;98(8):e0085824. doi: 10.1128/jvi.00858-24. Epub 2024 Jul 30. J Virol. 2024. PMID: 39078257
-
Hypoxia inducible factors inhibit respiratory syncytial virus infection by modulation of nucleolin expression.iScience. 2023 Dec 18;27(1):108763. doi: 10.1016/j.isci.2023.108763. eCollection 2024 Jan 19. iScience. 2023. PMID: 38261926 Free PMC article.
-
A Balancing Act: The Viral-Host Battle over RNA Binding Proteins.Viruses. 2024 Mar 20;16(3):474. doi: 10.3390/v16030474. Viruses. 2024. PMID: 38543839 Free PMC article. Review.
References
-
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.G. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

