A mouse model and pathogenesis study for CVA19 first isolated from hand, foot, and mouth disease
- PMID: 36735880
- PMCID: PMC9937014
- DOI: 10.1080/22221751.2023.2177084
A mouse model and pathogenesis study for CVA19 first isolated from hand, foot, and mouth disease
Abstract
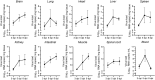
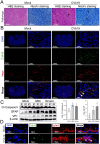
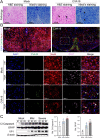
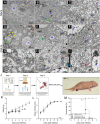
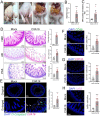
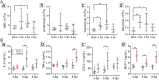
ABSTRACTCoxsackievirus A19 (CVA19) is a member of Enterovirus (EV) C group in the Picornaviridae family. Recently, we reported a case of CVA19-infected hand, foot, and mouth disease (HFMD) for the first time. However, the current body of knowledge on the CVA19 infection, particularly the pathogenesis of encephalomyelitis and diarrhoea is still very limited, due to the lack of suitable animal models. Here, we successfully established a CVA19 mouse model via oral route based on 7-day-old ICR mice. Our results found the virus strain could directly infect the neurons, astrocytes of brain, and motor neurons of spinal cord causing neurological complications, such as acute flaccid paralysis. Importantly, viruses isolated from the spinal cords of infected mice caused severe illness in suckling mice, fulfilling Koch's postulates to some extent. CVA19 infection led to diarrhoea with typical pathological features of shortened intestinal villi, increased number of secretory cells and apoptotic intestinal cells, and inflammatory cell infiltration. Much higher concentrations of serum cytokines and more peripheral blood inflammatory cells in CVA19-infected mice indicated a systematic inflammatory response induced by CVA19 infection. Finally, we found ribavirin and CVA19 VP1 monoclonal antibody could not prevent the disease progression, but higher concentrations of antisera and interferon alpha 2 (IFN-α2) could provide protective effects against CVA19. In conclusion, this study shows that a natural mouse-adapted CVA19 strain leads to diarrhoea and encephalomyelitis in a mouse model via oral infection, which provides a useful tool for studying CVA19 pathogenesis and evaluating the efficacy of vaccines and antivirals.
Keywords: Coxsackievirus A19; and mouth disease; diarrhoea; encephalomyelitis; foot; hand; oral infection.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
A Neonatal Murine Model of Coxsackievirus A6 Infection for Evaluation of Antiviral and Vaccine Efficacy.J Virol. 2017 Apr 13;91(9):e02450-16. doi: 10.1128/JVI.02450-16. Print 2017 May 1. J Virol. 2017. PMID: 28250116 Free PMC article.
-
A mouse-adapted CVA6 strain exhibits neurotropism and triggers systemic manifestations in a novel murine model.Emerg Microbes Infect. 2022 Dec;11(1):2248-2263. doi: 10.1080/22221751.2022.2119166. Emerg Microbes Infect. 2022. PMID: 36036059 Free PMC article.
-
A novel orally infected hamster model for Coxsackievirus A16 hand-foot-and-mouth disease and encephalomyelitis.Lab Invest. 2020 Sep;100(9):1262-1275. doi: 10.1038/s41374-020-0456-x. Epub 2020 Jun 29. Lab Invest. 2020. PMID: 32601355
-
Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease.Expert Rev Vaccines. 2018 Sep;17(9):819-831. doi: 10.1080/14760584.2018.1510326. Epub 2018 Aug 22. Expert Rev Vaccines. 2018. PMID: 30095317 Review.
-
Recent progress and challenges in drug development to fight hand, foot and mouth disease.Expert Opin Drug Discov. 2020 Mar;15(3):359-371. doi: 10.1080/17460441.2019.1659241. Epub 2019 Aug 30. Expert Opin Drug Discov. 2020. PMID: 31470744 Review.
Cited by
-
The key mechanisms of multi-system responses triggered by central nervous system damage in hand, foot, and mouth disease severity.Infect Med (Beijing). 2024 Jul 27;3(3):100124. doi: 10.1016/j.imj.2024.100124. eCollection 2024 Sep. Infect Med (Beijing). 2024. PMID: 39314804 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
