Extracellular vesicle-transmitted miR-671-5p alleviates lung inflammation and injury by regulating the AAK1/NF-κB axis
- PMID: 36733250
- PMCID: PMC10188640
- DOI: 10.1016/j.ymthe.2023.01.025
Extracellular vesicle-transmitted miR-671-5p alleviates lung inflammation and injury by regulating the AAK1/NF-κB axis
Abstract
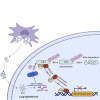
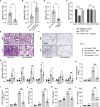
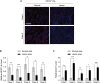
Mesenchymal stem cells regulate remote intercellular signaling communication via their secreted extracellular vesicles. Here, we report that menstrual blood-derived stem cells alleviate acute lung inflammation and injury via their extracellular vesicle-transmitted miR-671-5p. Disruption of this abundantly expressed miR-671-5p dramatically reduced the ameliorative effect of extracellular vesicles released by menstrual blood-derived stem cells on lipopolysaccharide (LPS)-induced pulmonary inflammatory injury. Mechanistically, miR-671-5p directly targets the kinase AAK1 for post-transcriptional degradation. AAK1 is found to positively regulate the activation of nuclear factor κB (NF-κB) signaling by controlling the stability of the inhibitory protein IκBα. This study identifies a potential molecular basis of how extracellular vesicles derived from mesenchymal stem cells improve pulmonary inflammatory injury and highlights the functional importance of the miR-671-5p/AAK1 axis in the progression of pulmonary inflammatory diseases. More importantly, this study provides a promising cell-based approach for the treatment of pulmonary inflammatory disorders through an extracellular vesicle-dependent pathway.
Keywords: AAK1; NF-κB signaling; extracellular vesicles; menstrual blood-derived stem cells; miR-671-5p; pulmonary inflammatory injury.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Small Extracellular Vesicles Secreted by iPSC-Derived MSCs Ameliorate Pulmonary Inflammation and Lung Injury Induced by Sepsis through Delivery of miR-125b-5p.J Immunol Res. 2023 Jul 1;2023:8987049. doi: 10.1155/2023/8987049. eCollection 2023. J Immunol Res. 2023. PMID: 37425491 Free PMC article.
-
Curcumin Alleviates Lipopolysaccharide (LPS)-Activated Neuroinflammation via Modulation of miR-199b-5p/IκB Kinase β (IKKβ)/Nuclear Factor Kappa B (NF-κB) Pathway in Microglia.Med Sci Monit. 2019 Dec 21;25:9801-9810. doi: 10.12659/MSM.918237. Med Sci Monit. 2019. PMID: 31862869 Free PMC article.
-
Extracellular Vesicles Induce Nuclear Factor-κB Activation and Interleukin-8 Synthesis through miRNA-191-5p Contributing to Inflammatory Processes: Potential Implications in the Pathogenesis of Chronic Obstructive Pulmonary Disease.Biomolecules. 2024 Aug 19;14(8):1030. doi: 10.3390/biom14081030. Biomolecules. 2024. PMID: 39199417 Free PMC article.
-
Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS-induced WI-38 cell apoptosis and inflammation in acute pneumonia.Life Sci. 2019 Jul 1;228:189-197. doi: 10.1016/j.lfs.2019.05.008. Epub 2019 May 7. Life Sci. 2019. PMID: 31071307 Review.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
Cited by
-
Recent progress in discovery of novel AAK1 inhibitors: from pain therapy to potential anti-viral agents.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2279906. doi: 10.1080/14756366.2023.2279906. Epub 2023 Nov 13. J Enzyme Inhib Med Chem. 2023. PMID: 37955299 Free PMC article. Review.
-
Enrichment of Bioactive Lipids in Urinary Extracellular Vesicles and Evidence of Apoptosis in Kidneys of Hypertensive Diabetic Cathepsin B Knockout Mice after Streptozotocin Treatment.Biomedicines. 2024 May 8;12(5):1038. doi: 10.3390/biomedicines12051038. Biomedicines. 2024. PMID: 38791000 Free PMC article.
-
The identification of key molecules and pathways in the crosstalk of calcium oxalate-treated TCMK-1 cells and macrophage via exosomes.Sci Rep. 2024 Sep 9;14(1):20949. doi: 10.1038/s41598-024-71755-y. Sci Rep. 2024. PMID: 39251681 Free PMC article.
-
Elevated extracellular particle concentration in plasma predicts in-hospital mortality after severe trauma.Front Immunol. 2024 Jun 12;15:1390380. doi: 10.3389/fimmu.2024.1390380. eCollection 2024. Front Immunol. 2024. PMID: 38933277 Free PMC article.
-
Identification of biomarkers associated with immune scores in diabetic retinopathy.Front Endocrinol (Lausanne). 2023 Oct 5;14:1228843. doi: 10.3389/fendo.2023.1228843. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37867507 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

