HIV vaccine candidate efficacy in female macaques mediated by cAMP-dependent efferocytosis and V2-specific ADCC
- PMID: 36732510
- PMCID: PMC9894672
- DOI: 10.1038/s41467-023-36109-8
HIV vaccine candidate efficacy in female macaques mediated by cAMP-dependent efferocytosis and V2-specific ADCC
Abstract
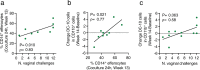
The development of an effective vaccine to protect against HIV acquisition will be greatly bolstered by in-depth understanding of the innate and adaptive responses to vaccination. We report here that the efficacy of DNA/ALVAC/gp120/alum vaccines, based on V2-specific antibodies mediating apoptosis of infected cells (V2-ADCC), is complemented by efferocytosis, a cyclic AMP (cAMP)-dependent antiphlogistic engulfment of apoptotic cells by CD14+ monocytes. Central to vaccine efficacy is the engagement of the CCL2/CCR2 axis and tolerogenic dendritic cells producing IL-10 (DC-10). Epigenetic reprogramming in CD14+ cells of the cyclic AMP/CREB pathway and increased systemic levels of miRNA-139-5p, a negative regulator of expression of the cAMP-specific phosphodiesterase PDE4D, correlated with vaccine efficacy. These data posit that efferocytosis, through the prompt and effective removal of apoptotic infected cells, contributes to vaccine efficacy by decreasing inflammation and maintaining tissue homeostasis.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no financial and non-financial competing interests. The US government has filed the patent, NIH (DHHS) Ref. No. E-160-2018-0-EP-05 on the HIV ΔV1-envelope immunogens.
Figures



Similar articles
-
Expression of CD40L by the ALVAC-Simian Immunodeficiency Virus Vector Abrogates T Cell Responses in Macaques.J Virol. 2020 Feb 28;94(6):e01933-19. doi: 10.1128/JVI.01933-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31896599 Free PMC article.
-
Cholera toxin B scaffolded, focused SIV V2 epitope elicits antibodies that influence the risk of SIVmac251 acquisition in macaques.Front Immunol. 2023 Apr 21;14:1139402. doi: 10.3389/fimmu.2023.1139402. eCollection 2023. Front Immunol. 2023. PMID: 37153584 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Nonneutralizing functional antibodies: a new "old" paradigm for HIV vaccines.Clin Vaccine Immunol. 2014 Aug;21(8):1023-36. doi: 10.1128/CVI.00230-14. Epub 2014 Jun 11. Clin Vaccine Immunol. 2014. PMID: 24920599 Free PMC article. Review.
-
Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses.Curr HIV Res. 2013 Jul;11(5):378-87. doi: 10.2174/1570162x113116660059. Curr HIV Res. 2013. PMID: 24191939 Free PMC article. Review.
Cited by
-
Loss of HIV candidate vaccine efficacy in male macaques by mucosal nanoparticle immunization rescued by V2-specific response.Nat Commun. 2024 Oct 22;15(1):9102. doi: 10.1038/s41467-024-53359-2. Nat Commun. 2024. PMID: 39438480 Free PMC article.
-
HIV vaccine candidate plus microbicide decreases SIV infection risk by more than 90.Nat Microbiol. 2023 May;8(5):767-768. doi: 10.1038/s41564-023-01367-1. Nat Microbiol. 2023. PMID: 37024619 No abstract available.
-
Innate protection against intrarectal SIV acquisition by a live SHIV vaccine.JCI Insight. 2024 May 21;9(12):e175800. doi: 10.1172/jci.insight.175800. JCI Insight. 2024. PMID: 38912579 Free PMC article.
-
Vaccine plus microbicide effective in preventing vaginal SIV transmission in macaques.Nat Microbiol. 2023 May;8(5):905-918. doi: 10.1038/s41564-023-01353-7. Epub 2023 Apr 6. Nat Microbiol. 2023. PMID: 37024617 Free PMC article.
-
In Vivo Treatment with Insulin-like Growth Factor 1 Reduces CCR5 Expression on Vaccine-Induced Activated CD4+ T-Cells.Vaccines (Basel). 2023 Oct 30;11(11):1662. doi: 10.3390/vaccines11111662. Vaccines (Basel). 2023. PMID: 38005994 Free PMC article.
References
-
- Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

