The dynamics of chromatin states mediated by epigenetic modifications during somatic cell reprogramming
- PMID: 36727112
- PMCID: PMC9884706
- DOI: 10.3389/fcell.2023.1097780
The dynamics of chromatin states mediated by epigenetic modifications during somatic cell reprogramming
Abstract
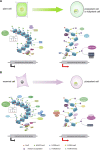
Somatic cell reprogramming (SCR) is the conversion of differentiated somatic cells into totipotent or pluripotent cells through a variety of methods. Somatic cell reprogramming also provides a platform to investigate the role of chromatin-based factors in establishing and maintaining totipotency or pluripotency, since high expression of totipotency- or pluripotency-related genes usually require an active chromatin state. Several studies in plants or mammals have recently shed light on the molecular mechanisms by which epigenetic modifications regulate the expression of totipotency or pluripotency genes by altering their chromatin states. In this review, we present a comprehensive overview of the dynamic changes in epigenetic modifications and chromatin states during reprogramming from somatic cells to totipotent or pluripotent cells. In addition, we illustrate the potential role of DNA methylation, histone modifications, histone variants, and chromatin remodeling during somatic cell reprogramming, which will pave the way to developing reliable strategies for efficient cellular reprogramming.
Keywords: cell pluripotency; cell totipotency; chromatin state; epigenetic modification; gene expression; somatic cell reprogramming.
Copyright © 2023 Peng, Zhang, Zhang, Su and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Epigenetic reprogramming in the transition from pluripotency to totipotency.J Cell Physiol. 2024 May;239(5):e31222. doi: 10.1002/jcp.31222. Epub 2024 Feb 20. J Cell Physiol. 2024. PMID: 38375873 Review.
-
Going up the hill: chromatin-based barriers to epigenetic reprogramming.FEBS J. 2021 Aug;288(16):4798-4811. doi: 10.1111/febs.15628. Epub 2020 Dec 6. FEBS J. 2021. PMID: 33190371 Review.
-
Transcriptional and epigenetic mechanisms of cellular reprogramming to induced pluripotency.Epigenomics. 2016 Aug;8(8):1131-49. doi: 10.2217/epi-2016-0032. Epub 2016 Jul 15. Epigenomics. 2016. PMID: 27419933 Free PMC article. Review.
-
Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2.Nature. 2012 Aug 30;488(7413):652-5. doi: 10.1038/nature11333. Nature. 2012. PMID: 22902501 Free PMC article.
-
Targeting cellular memory to reprogram the epigenome, restore potential, and improve somatic cell nuclear transfer.Anim Reprod Sci. 2007 Mar;98(1-2):129-46. doi: 10.1016/j.anireprosci.2006.10.019. Epub 2006 Oct 21. Anim Reprod Sci. 2007. PMID: 17166676 Review.
Cited by
-
Involvement of the H3.3 Histone Variant in the Epigenetic Regulation of Gene Expression in the Nervous System, in Both Physiological and Pathological Conditions.Int J Mol Sci. 2023 Jul 3;24(13):11028. doi: 10.3390/ijms241311028. Int J Mol Sci. 2023. PMID: 37446205 Free PMC article. Review.
-
Small molecules, enormous functions: potential approach for overcoming bottlenecks in embryogenic tissue induction and maintenance in conifers.Hortic Res. 2024 Jul 10;11(8):uhae180. doi: 10.1093/hr/uhae180. eCollection 2024 Aug. Hortic Res. 2024. PMID: 39108576 Free PMC article. Review.
References
-
- Anzola J. M., Sieberer T., Ortbauer M., Butt H., Korbei B., Weinhofer I., et al. (2010). Putative Arabidopsis transcriptional adaptor protein (PROPORZ1) is required to modulate histone acetylation in response to auxin. Proc. Natl. Acad. Sci. U. S. A. 107 (22), 10308–10313. 10.1073/pnas.0913918107 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

