Interval time between neoadjuvant chemotherapy and surgery in advanced gastric cancer doesn't affect outcome: A meta analysis
- PMID: 36726960
- PMCID: PMC9885804
- DOI: 10.3389/fsurg.2022.1047456
Interval time between neoadjuvant chemotherapy and surgery in advanced gastric cancer doesn't affect outcome: A meta analysis
Abstract
Background: The efficacy of neoadjuvant chemotherapy for advanced gastric cancer is not yet firmly confirmed, but the exciting results demonstrated in several clinical studies have led neoadjuvant chemotherapy as the important treatment methods in guidelines. The 4-6 weeks interval time is currently the most commonly used in clinical treatment, but there are insufficient studies to support this time and the optimal interval has not yet been identified. The aim of this meta-analysis was to investigate the short-term life quality and long-term prognostic impact of the interval time between the end of neoadjuvant chemotherapy and surgery in patients with advanced gastric cancer.
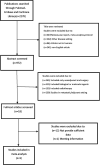
Methods: We conducted a systematic literature search in PUBMED, Embase and Cochrane Liabrary for studies published or reported in English from January 2006 to May 2022. We summarised relevant studies for the time to surgery (TTS), included as retrospective studies and prospective studies. The primary study outcome was the rate of pathological complete response (pCR), and the secondary outcomes included R0 resection rate, incidence of serious postoperative complications, 3-year progression free survival time (PFS) rate and overall survival time (OS) rate. TTS were classified in three groups: 4-6 weeks, <4 weeks and >6 weeks. The ratio ratios (ORs) were calculated and forest plots and funnel plots were made to analysis by using fixed-effect and random-effect models in Review Manager 5.2.
Results: A total of five studies included 1,171 patients: 411 patients in shorter TTS group (<4 weeks), 507 patients in medium TTS group (4-6 weeks) and 253 patients in longer TTS groups (>6 weeks). And The results of our meta-analysis indicate that there are no significant difference between the three groups. The pCR, R0 resection rate, incidence of serious postoperative complications, 3-year PFS and OS were similar between three groups.
Conclusions: Although there many studies exploring the suitable TTS in advanced gastric cancer, but we have not find the evidence to prove the TTS is the risk factor influencing the outcome.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022369009.
Keywords: advanced gastric cancer; interval time; neoadjuvant chemotherapy; pathological complete response; surgery.
© 2023 Zhai, Zheng, Deng, Yin, Bai, Liu, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Optimal timing of surgery for gastric cancer after neoadjuvant chemotherapy: a systematic review and meta-analysis.World J Surg Oncol. 2023 Dec 1;21(1):377. doi: 10.1186/s12957-023-03251-y. World J Surg Oncol. 2023. PMID: 38037067 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies.Dis Esophagus. 2016 Nov;29(8):1107-1114. doi: 10.1111/dote.12432. Epub 2015 Nov 6. Dis Esophagus. 2016. PMID: 26542065
-
Time to surgery does not affect oncologic outcomes in locally advanced gastric cancer after neoadjuvant chemotherapy: a meta-analysis.Future Oncol. 2023 Feb;19(5):397-408. doi: 10.2217/fon-2022-1061. Epub 2023 Mar 15. Future Oncol. 2023. PMID: 36919890
-
Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis.Eur Urol. 2014 Feb;65(2):350-7. doi: 10.1016/j.eururo.2013.06.049. Epub 2013 Jul 3. Eur Urol. 2014. PMID: 23849998 Review.
Cited by
-
Textbook Neoadjuvant Outcome-Novel Composite Measure of Oncological Outcomes among Gastric Cancer Patients Undergoing Multimodal Treatment.Cancers (Basel). 2024 Apr 28;16(9):1721. doi: 10.3390/cancers16091721. Cancers (Basel). 2024. PMID: 38730672 Free PMC article. Review.
-
Chemotherapy-associated pneumoperitoneum in cancer patients: a scoping review.Ann Med Surg (Lond). 2024 Mar 25;86(5):2828-2835. doi: 10.1097/MS9.0000000000001998. eCollection 2024 May. Ann Med Surg (Lond). 2024. PMID: 38694333 Free PMC article.
-
Patients Undergoing Systemic Anti-Cancer Therapy Who Require Surgical Intervention: What Surgeons Need to Know.Cancers (Basel). 2023 Jul 26;15(15):3781. doi: 10.3390/cancers15153781. Cancers (Basel). 2023. PMID: 37568597 Free PMC article. Review.
References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology[M/OL]: Gastric Cancer. Ver 3. (2020). [2020-8-14] http://www.nccn.org
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

