Neuroregeneration of injured peripheral nerve by fraction B of catfish epidermal secretions through the reversal of the apoptotic pathway and DNA damage
- PMID: 36726586
- PMCID: PMC9885176
- DOI: 10.3389/fphar.2023.1085314
Neuroregeneration of injured peripheral nerve by fraction B of catfish epidermal secretions through the reversal of the apoptotic pathway and DNA damage
Abstract
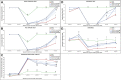
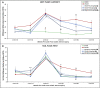
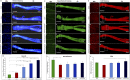
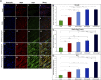
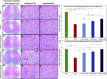
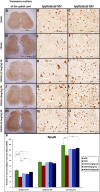
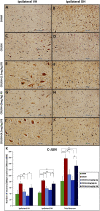
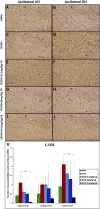
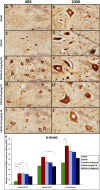
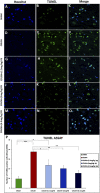
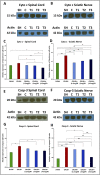
Introduction: Crush injuries occur from acute traumatic nerve compression resulting in different degrees of neural damage leading to permanent functional deficits. Recently, we have shown that administration of Fraction B (FB) derived from catfish epidermal secretions accelerates healing of damaged nerve in a sciatic nerve crush injury, as it ameliorates the neurobehavioral deficits and enhances axonal regeneration, as well as protects spinal neurons and increases astrocytic activity and decreasing GAP-43 expression. The present study aimed to investigate the role of FB treatment on the apoptotic pathway in the neuroregeneration of the sciatic nerve crush injury. Methods: Male Wistar rats were randomly assigned into five groups: (I) SHAM, (II) CRUSH, (III) CRUSH + (1.5 mg/kg) FB, (IV) CRUSH + (3 mg/kg) FB, and (V) CRUSH + (4.5 mg/kg) FB. Rats underwent sciatic nerve crush surgery, followed by treatment with FB administered intraperitoneally (IP) daily for two weeks and then sacrificed at the end of the fourth week. Results: FB improved the recovery of neurobehavioral functions with a concomitant increase in axonal regeneration and neuroprotective effects on spinal cord neurons following crush injury. Further, FB enhanced Schwann cells (SCs) proliferation with a significant increase in myelin basic protein expression. FB-treated animals demonstrated higher numbers of neurons in the spinal cord, possibly through ameliorating oxidative DNA damage and alleviating the mitochondrial-dependent apoptotic pathway by inhibiting the release of cytochrome c and the activation of caspase-3 in the spinal cord neurons. Conclusion: FB alleviates the neurodegenerative changes in the lumbar spinal cord neurons and recovers the decrease in the neuronal count through its anti-apoptotic and DNA antioxidative properties.
Keywords: apoptosis; catfish fraction B; nerve injury; neuroprotection; neuroregeneration.
Copyright © 2023 Al-Arbeed, Renno and Al-Hassan.
Conflict of interest statement
JMH and WMR hold patents on the use of products from the catfish gel for treating various diseases. TA declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Catfish Epidermal Preparation Accelerates Healing of Damaged Nerve in a Sciatic Nerve Crush Injury Rat Model.Front Pharmacol. 2021 Apr 14;12:632028. doi: 10.3389/fphar.2021.632028. eCollection 2021. Front Pharmacol. 2021. PMID: 33986668 Free PMC article.
-
Possible role of antioxidative capacity of (-)-epigallocatechin-3-gallate treatment in morphological and neurobehavioral recovery after sciatic nerve crush injury.J Neurosurg Spine. 2017 Nov;27(5):593-613. doi: 10.3171/2016.10.SPINE16218. Epub 2017 Aug 4. J Neurosurg Spine. 2017. PMID: 28777065
-
Monoamine oxidase-B inhibitor protects degenerating spinal neurons, enhances nerve regeneration and functional recovery in sciatic nerve crush injury model.Neuropharmacology. 2018 Jan;128:231-243. doi: 10.1016/j.neuropharm.2017.10.020. Epub 2017 Oct 17. Neuropharmacology. 2018. PMID: 29054367
-
(-)-Epigallocatechin-3-gallate modulates spinal cord neuronal degeneration by enhancing growth-associated protein 43, B-cell lymphoma 2, and decreasing B-cell lymphoma 2-associated x protein expression after sciatic nerve crush injury.J Neurotrauma. 2015 Feb 1;32(3):170-84. doi: 10.1089/neu.2014.3491. Epub 2014 Nov 10. J Neurotrauma. 2015. PMID: 25025489 Free PMC article.
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
Cited by
-
Oxidized Cholesterol Derivatives in Fraction B Prepared from Gulf Catfish (Arius bilineatus, Val.) Skin Regulate Calcium Response and Neutrophil Extracellular Traps (NETs) Formation.Biomedicines. 2024 Jun 21;12(7):1380. doi: 10.3390/biomedicines12071380. Biomedicines. 2024. PMID: 39061953 Free PMC article.
References
-
- Al-Hassan J. M. (1990). Diabetic ulcer healing preparations from the skin of the arabian Gulf catfish (Arius bilineatus val.): A novel and effective treatment. Int. J. Tissue React. 12 (2), 121–135. - PubMed
-
- Al-Hassan J. M., Dyson M., Young S. R., Thomson M., Criddle R. S. (1991). Acceleration of wound healing responses induced by preparations from the epidermal secretions of the Arabian Gulf catfish (Arius bilineatus, Valenciennes). J. Wilderness Med. 2, 153–163. 10.1580/0953-9859-2.3.153 - DOI
-
- Al-Hassan J. M., Hinek A., Renno W. M., Wang Y., Liu Y. F., Guan R., et al. (2020). Potential mechanism of dermal wound treatment with preparations from the skin gel of arabian Gulf catfish: A unique furan fatty acid (F6) and cholesta-3, 5-diene (S5) recruit neutrophils and fibroblasts to promote wound healing. Front. Pharmacol. 11, 899. 10.3389/fphar.2020.00899 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

