Triple therapy in COPD: understanding the data
- PMID: 36726367
- PMCID: PMC9885273
- DOI: 10.1183/23120541.00615-2022
Triple therapy in COPD: understanding the data
Abstract
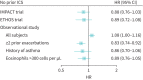
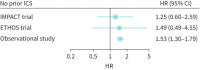
Effectiveness of single-inhaler triple therapy on exacerbation risk and mortality in COPD is exaggerated in IMPACT and ETHOS trials from confounding by prior ICS discontinuation: effectiveness fades in analyses and studies with no prior ICS discontinuation https://bit.ly/3tOgNdW.
Copyright ©The authors 2023.
Conflict of interest statement
Provenance: Submitted article, peer reviewed. Conflict of interest: The author attended scientific advisory committee meetings or received speaking fees from AstraZeneca, Atara, Boehringer Ingelheim, Bristol-Myers-Squibb, Merck, Novartis, Panalgo, Pfizer and Seqirus.
Figures
Similar articles
-
Single-inhaler triple vs single-inhaler dual therapy in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized control trials.Respir Res. 2021 Jul 23;22(1):209. doi: 10.1186/s12931-021-01794-w. Respir Res. 2021. PMID: 34301267 Free PMC article.
-
Single-Inhaler Triple versus Dual Bronchodilator Therapy in COPD: Real-World Comparative Effectiveness and Safety.Int J Chron Obstruct Pulmon Dis. 2022 Aug 30;17:1975-1986. doi: 10.2147/COPD.S378486. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36065315 Free PMC article.
-
A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6 μg and 160/18/9.6 μg using co-suspension delivery technology in moderate-to-very severe COPD: The ETHOS study protocol.Respir Med. 2019 Oct-Nov;158:59-66. doi: 10.1016/j.rmed.2019.08.010. Epub 2019 Aug 22. Respir Med. 2019. PMID: 31605923 Clinical Trial.
-
The Impact of 52-Week Single Inhaler Device Triple Therapy versus Dual Therapy on the Mortality of COPD Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Life (Basel). 2022 Jan 25;12(2):173. doi: 10.3390/life12020173. Life (Basel). 2022. PMID: 35207460 Free PMC article. Review.
-
Current appraisal of single inhaler triple therapy in COPD.Int J Chron Obstruct Pulmon Dis. 2018 Sep 28;13:3003-3009. doi: 10.2147/COPD.S177333. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30319248 Free PMC article. Review.
Cited by
-
Comorbidities in COPD: Current and Future Treatment Challenges.J Clin Med. 2024 Jan 27;13(3):743. doi: 10.3390/jcm13030743. J Clin Med. 2024. PMID: 38337438 Free PMC article. Review.
-
Optimizing Pharmacotherapy Management of Chronic Obstructive Pulmonary Disease: Don't Miss the Forest for the Trees.Am J Respir Crit Care Med. 2024 Sep 1;210(5):545-547. doi: 10.1164/rccm.202402-0338VP. Am J Respir Crit Care Med. 2024. PMID: 38626371 Free PMC article. No abstract available.
-
Inhaled Corticosteroids in Subjects with Chronic Obstructive Pulmonary Disease: An Old, Unfinished History.Biomolecules. 2024 Feb 6;14(2):195. doi: 10.3390/biom14020195. Biomolecules. 2024. PMID: 38397432 Free PMC article. Review.
-
The Current Molecular and Cellular Landscape of Chronic Obstructive Pulmonary Disease (COPD): A Review of Therapies and Efforts towards Personalized Treatment.Proteomes. 2024 Aug 16;12(3):23. doi: 10.3390/proteomes12030023. Proteomes. 2024. PMID: 39189263 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
