Cryptotanshinone attenuates LPS-induced acute lung injury by regulating metabolic reprogramming of macrophage
- PMID: 36714100
- PMCID: PMC9880059
- DOI: 10.3389/fmed.2022.1075465
Cryptotanshinone attenuates LPS-induced acute lung injury by regulating metabolic reprogramming of macrophage
Abstract
Background: Acute lung injury (ALI) is a life-threatening inflammatory disease without effective therapeutic regimen. Macrophage polarization plays a key role in the initiation and resolution of pulmonary inflammation. Therefore, modulating macrophage phenotype is a potentially effective way for acute lung injury. Cryptotanshinone (CTS) is a lipophilic bioactive compound extracted from the root of Salvia miltiorrhiza with a variety of pharmacological effects, especially the anti-inflammatory role. In this study, we investigated the therapeutic and immunomodulatory effects of CTS on ALI.
Materials and methods: The rat model of ALI was established by intratracheal instillation of LPS (5 mg/kg) to evaluate the lung protective effect of CTS in vivo and to explore the regulation of CTS on the phenotype of lung macrophage polarization. LPS (1 μg/mL) was used to stimulate RAW264.7 macrophages in vitro to further explore the effect of CTS on the polarization and metabolic reprogramming of RAW264.7 macrophages and to clarify the potential mechanism of CTS anti-ALI.
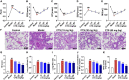
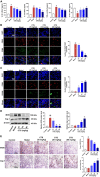
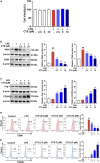
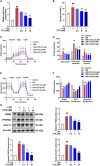
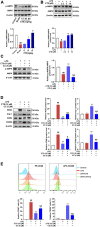
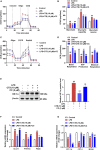
Results: CTS significantly improved lung function, reduced pulmonary edema, effectively inhibited pulmonary inflammatory infiltration, and alleviated ALI. Both in vivo and in vitro results revealed that CTS inhibited the differentiation of macrophage into the M1 phenotype and promoted polarization into M2 phenotype during ALI. Further in vitro studies indicated that CTS significantly suppressed LPS-induced metabolic transition from aerobic oxidation to glycolysis in macrophages. Mechanistically, CTS blocked LPS-induced metabolic transformation of macrophages by activating AMPK.
Conclusion: These findings demonstrated that CTS regulates macrophage metabolism by activating AMPK, and then induced M1-type macrophages to transform into M2-type macrophages, thereby alleviating the inflammatory response of ALI, suggesting that CTS might be a potential anti-ALI agent.
Keywords: AMPK; Cryptotanshinone; acute lung injury; macrophage polarization; metabolic reprogramming.
Copyright © 2023 Ye, Wang, Feng, Wang, Liu, Lu, Chen and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Intermittent fasting attenuates lipopolysaccharide-induced acute lung injury in mice by modulating macrophage polarization.J Nutr Biochem. 2022 Dec;110:109133. doi: 10.1016/j.jnutbio.2022.109133. Epub 2022 Aug 24. J Nutr Biochem. 2022. PMID: 36028098
-
[Baicalin alleviates LPS-induced acute lung injury in rats by regulating macrophage polarization].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022 Jan;38(1):9-15. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022. PMID: 35078570 Chinese.
-
MEF2 intervened LPS-induced acute lung injury by binding to KLF2 promoter and modulating macrophage phenotype.Int Immunopharmacol. 2022 Jul;108:108873. doi: 10.1016/j.intimp.2022.108873. Epub 2022 Jun 3. Int Immunopharmacol. 2022. PMID: 35729843
-
The role of immunometabolism in macrophage polarization and its impact on acute lung injury/acute respiratory distress syndrome.Front Immunol. 2023 Mar 20;14:1117548. doi: 10.3389/fimmu.2023.1117548. eCollection 2023. Front Immunol. 2023. PMID: 37020557 Free PMC article. Review.
-
[Research progress on polarization regulation mechanism of alveolar macrophages in acute lung injury].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020 Oct;32(10):1269-1272. doi: 10.3760/cma.j.cn121430-20200426-00335. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020. PMID: 33198879 Review. Chinese.
Cited by
-
Macrophage polarization and its impact on idiopathic pulmonary fibrosis.Front Immunol. 2024 Jul 26;15:1444964. doi: 10.3389/fimmu.2024.1444964. eCollection 2024. Front Immunol. 2024. PMID: 39131154 Free PMC article. Review.
-
Impact of time intervals on drug efficacy and phenotypic outcomes in acute respiratory distress syndrome in mice.Sci Rep. 2024 Sep 5;14(1):20768. doi: 10.1038/s41598-024-71659-x. Sci Rep. 2024. PMID: 39237657 Free PMC article.
-
Macrophage‑driven pathogenesis in acute lung injury/acute respiratory disease syndrome: Harnessing natural products for therapeutic interventions (Review).Mol Med Rep. 2025 Jan;31(1):16. doi: 10.3892/mmr.2024.13381. Epub 2024 Nov 8. Mol Med Rep. 2025. PMID: 39513609 Free PMC article. Review.
-
Fibroblast Growth Factor 21 Relieves Lipopolysaccharide-Induced Acute Lung Injury by Suppressing JAK2/STAT3 Signaling Pathway.Inflammation. 2024 Feb;47(1):209-226. doi: 10.1007/s10753-023-01905-3. Epub 2023 Oct 21. Inflammation. 2024. PMID: 37864659 Free PMC article.
-
Cryptotanshinone affects HFL-1 cells proliferation by inhibiting cytokines secretion in RAW264.7 cells and ameliorates inflammation and fibrosis in newborn rats with hyperoxia induced lung injury.Front Pharmacol. 2023 Jul 25;14:1192370. doi: 10.3389/fphar.2023.1192370. eCollection 2023. Front Pharmacol. 2023. PMID: 37560477 Free PMC article.
References
-
- Zhu J, Guo M, Cui Y, Meng Y, Ding J, Zeng W, et al. Surface coating of pulmonary siRNA delivery vectors enabling mucus penetration, cell targeting, and intracellular radical scavenging for enhanced acute lung injury therapy. ACS Appl Mater Interfaces. (2022) 14:5090–100. 10.1021/acsami.1c23069 - DOI - PubMed
-
- Nieman G, Gatto L, Andrews P, Satalin J, Camporota L, Daxon B, et al. Prevention and treatment of acute lung injury with time-controlled adaptive ventilation: physiologically informed modification of airway pressure release ventilation. Ann Intensive Care. (2020) 10:3. 10.1186/s13613-019-0619-3 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

