Effect of female coronavirus disease 2019 vaccination on assisted reproductive outcomes: a systematic review and meta-analysis
- PMID: 36702343
- PMCID: PMC9868006
- DOI: 10.1016/j.fertnstert.2023.01.024
Effect of female coronavirus disease 2019 vaccination on assisted reproductive outcomes: a systematic review and meta-analysis
Abstract
Importance: The effect of coronavirus disease 2019 (COVID-19) vaccination on fertility warrants clarification in women undergoing assisted reproductive treatment.
Objective: To study the association between female COVID-19 vaccination and outcomes of assisted reproductive treatment.
Data sources: PubMed, Embase, the Web of Science, Cochrane Library, and medRxiv and bioRxiv were searched for eligible studies from December 1, 2019, to November 30, 2022, with no language restrictions.
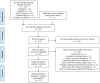
Study selection and synthesis: Observational studies comparing assisted reproductive outcomes between women with and without COVID-19 vaccination were included. The pooled estimates were calculated using the random-effects models as mean differences (MDs), standardized MDs, or odds ratios with 95% confidence intervals (CIs). Heterogeneity was evaluated using the I2 statistic.
Main outcomes: The number of oocytes retrieved and clinical pregnancy rate.
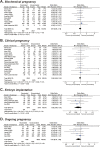
Results: Twenty-one cohort studies involving a total of 19,687 treatment cycles were included. In a comparison of the vaccinated vs. unvaccinated groups, the pooled MD for oocyte number was -0.06 (95% CI, -0.51 to 0.39; I2 = 0), and the pooled odds ratio for clinical pregnancy was 0.95 (95% CI, 0.85-1.05; I2 = 0). Similarly, there were no statistically significant adverse effects identified in other outcomes determined a priori, including 4 cycle characteristics, 6 laboratory parameters, and 3 pregnancy indicators. Most results were consistently unchanged in subgroup and sensitivity analyses, with no evidence of publication bias according to Egger's test.
Conclusion and relevance: Our work did not find significant differences in assisted reproductive outcomes between vaccinated and unvaccinated women. However, more data are warranted to confirm the safety of COVID-19 vaccination for assisted reproductive treatment and in female fertility in general.
Efectos de la vacunación femenina para la enfermedad del coronavirus 2019 en los resultados de reproducción asistida: revisión sistemática y meta-análisis.
Importancia: el efecto de la vacunación para la enfermedad del coronavirus 2019 (COVID-19) en la fertilidad merece una aclaración en mujeres que se someten a un tratamiento de reproducción asistida.
Objetivo: Estudiar la asociación entre la vacunación contra el COVID-19 y los resultados de los tratamientos de reproducción asistida.
Fuentes de la data: Se buscaron estudios elegibles en PubMed, Embase, la web de la ciencia, Librería Cochrane, y medRxiv y bioRxiv desde Diciembre 1, 2019 a Noviembre 30, 2022, sin restricciones en el idioma.
Selección del estudio y síntesis: Se incluyeron estudios observacionales comparando los resultados de reproducción asistida entre mujeres con o sin vacunación contra COVID-19. La estimación agrupada fue calculada usando los modelos aleatorios-efectos como diferencias media (MDs), MDs estandarizada, o con razón de momios con 95% de intervalo de confianza (CIs). La heterogeneidad fue valorada usando estadista l2.
Resultados principales: número de óvulos recuperados y tasa de embarazo clínico.
Resultados: Se incluyeron veinte y un estudios de cohorte incluyendo un total de 19.687 ciclos de tratamiento. En una comparación de los grupos vacunados vs no vacunados, la MD agrupadas para óvulos maduros fue 0.06 (95% CI_0.51 a 0.39;I2 ¼ 0), y la razón de momios agrupadas para embarazo clínico fue 0.95 (95% CI, 0.85-1.05; I2 ¼ 0). De forma similar no hubo diferencias estadísticamente significativas en la identificación de efectos adversos en otros resultados determinados a priori, incluyendo 4 características de ciclos, 6 parámetros de laboratorio y 3 indicadores de embarazo. Muchos resultados fueron consistentemente sin cambios en los análisis sensitivos y subgrupales, sin evidencia de bias en la publicación acorde al test de Egger’s.
Conclusiones y relevancia: Nuestro trabajo no encontró diferencias significativas entre los resultados de reproducción asistida de mujeres vacunadas y no vacunadas. Sin embrago, se necesitan más datos para confirmar la seguridad de la vacunación contra COVID -19 para los tratamientos de reproducción asistida y para las fertilidad femenina en general.
Keywords: COVID-19; SARS-CoV-2; assisted reproductive technology; fertility; vaccine.
Copyright © 2023. Published by Elsevier Inc.
Figures




Comment in
-
Correspondence on "Effect of female COVID-19 vaccination on assisted reproductive outcomes: a systematic review and meta-analysis".Fertil Steril. 2023 Jun;119(6):1087. doi: 10.1016/j.fertnstert.2023.02.017. Epub 2023 Feb 17. Fertil Steril. 2023. PMID: 36805043 Free PMC article. No abstract available.
-
Coronavirus disease 2019 vaccination in women and assisted reproductive technology.Fertil Steril. 2023 May;119(5):784. doi: 10.1016/j.fertnstert.2023.03.022. Epub 2023 Mar 23. Fertil Steril. 2023. PMID: 36965597 Free PMC article. No abstract available.
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Coronavirus Disease 2019 (COVID-19) Vaccination and Assisted Reproduction Outcomes: A Systematic Review and Meta-analysis.Obstet Gynecol. 2024 Feb 1;143(2):210-218. doi: 10.1097/AOG.0000000000005310. Epub 2023 Jul 13. Obstet Gynecol. 2024. PMID: 37441788
-
Effect of COVID-19 vaccination on semen parameters: A systematic review and meta-analysis.J Med Virol. 2023 Jan;95(1):e28263. doi: 10.1002/jmv.28263. Epub 2022 Nov 8. J Med Virol. 2023. PMID: 36310390 Free PMC article.
-
Metabolomics for improving pregnancy outcomes in women undergoing assisted reproductive technologies.Cochrane Database Syst Rev. 2017 May 23;5(5):CD011872. doi: 10.1002/14651858.CD011872.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Mar 16;3:CD011872. doi: 10.1002/14651858.CD011872.pub3. PMID: 28534597 Free PMC article. Updated. Review.
-
Autologous platelet-rich plasma for assisted reproduction.Cochrane Database Syst Rev. 2024 Apr 29;4(4):CD013875. doi: 10.1002/14651858.CD013875.pub2. Cochrane Database Syst Rev. 2024. PMID: 38682756 Review.
Cited by
-
Correspondence on "Effect of female COVID-19 vaccination on assisted reproductive outcomes: a systematic review and meta-analysis".Fertil Steril. 2023 Jun;119(6):1087. doi: 10.1016/j.fertnstert.2023.02.017. Epub 2023 Feb 17. Fertil Steril. 2023. PMID: 36805043 Free PMC article. No abstract available.
-
Coronavirus disease 2019 vaccination in women and assisted reproductive technology.Fertil Steril. 2023 May;119(5):784. doi: 10.1016/j.fertnstert.2023.03.022. Epub 2023 Mar 23. Fertil Steril. 2023. PMID: 36965597 Free PMC article. No abstract available.
-
Assisted reproductive technology in Japan: A summary report for 2021 by the Ethics Committee of the Japan Society of Obstetrics and Gynecology.Reprod Med Biol. 2023 Dec 30;23(1):e12552. doi: 10.1002/rmb2.12552. eCollection 2024 Jan-Dec. Reprod Med Biol. 2023. PMID: 38163009 Free PMC article.
-
COVID-19 and abnormal uterine bleeding: potential associations and mechanisms.Clin Sci (Lond). 2024 Feb 21;138(4):153-171. doi: 10.1042/CS20220280. Clin Sci (Lond). 2024. PMID: 38372528 Free PMC article. Review.
-
Effects of the Severe Acute Respiratory Syndrome Coronavirus 2 Inactivated Vaccine on the Outcome of Frozen Embryo Transfers: A Large Scale Clinical Study.Int J Womens Health. 2023 Aug 8;15:1305-1316. doi: 10.2147/IJWH.S407773. eCollection 2023. Int J Womens Health. 2023. PMID: 37576183 Free PMC article.
References
-
- World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

