Cytosolic and mitochondrial ribosomal proteins mediate the locust phase transition via divergence of translational profiles
- PMID: 36701367
- PMCID: PMC9945961
- DOI: 10.1073/pnas.2216851120
Cytosolic and mitochondrial ribosomal proteins mediate the locust phase transition via divergence of translational profiles
Abstract
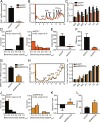
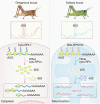
The phase transition from solitary to gregarious locusts is crucial in outbreaks of locust plague, which threaten agricultural yield and food security. Research on the regulatory mechanisms of phase transition in locusts has focused primarily on the transcriptional or posttranslational level. However, the translational regulation of phase transition is unexplored. Here, we show a phase-dependent pattern at the translation level, which exhibits different polysome profiles between gregarious and solitary locusts. The gregarious locusts exhibit significant increases in 60S and polyribosomes, while solitary locusts possess higher peaks of the monoribosome and a specific "halfmer." The polysome profiles, a molecular phenotype, respond to changes in population density. In gregarious locusts, ten genes involved in the cytosolic ribosome pathway exhibited increased translational efficiency (TE). In solitary locusts, five genes from the mitochondrial ribosome pathway displayed increased TE. The high expression of large ribosomal protein 7 at the translational level promotes accumulation of the free 60S ribosomal subunit in gregarious locusts, while solitary locusts employ mitochondrial small ribosomal protein 18c to induce the assembly of mitochondrial ribosomes, causing divergence of the translational profiles and behavioral transition. This study reveals the translational regulatory mechanism of locust phase transition, in which the locusts employ divergent ribosome pathways to cope with changes in population density.
Keywords: behavioral aggregation; cytosolic and mitochondrial ribosomal proteins; migratory locust; phase transition; translational regulation.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
4-Vinylanisole promotes conspecific interaction and acquisition of gregarious behavior in the migratory locust.Proc Natl Acad Sci U S A. 2023 Sep 12;120(37):e2306659120. doi: 10.1073/pnas.2306659120. Epub 2023 Sep 5. Proc Natl Acad Sci U S A. 2023. PMID: 37669362 Free PMC article.
-
Altered immunity in crowded locust reduced fungal (Metarhizium anisopliae) pathogenesis.PLoS Pathog. 2013 Jan;9(1):e1003102. doi: 10.1371/journal.ppat.1003102. Epub 2013 Jan 10. PLoS Pathog. 2013. PMID: 23326229 Free PMC article.
-
Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits.Proc Natl Acad Sci U S A. 2022 Jan 4;119(1):e2115753118. doi: 10.1073/pnas.2115753118. Proc Natl Acad Sci U S A. 2022. PMID: 34969848 Free PMC article.
-
Phenotypic Plasticity in Locusts: Trade-Off Between Migration and Reproduction.Annu Rev Entomol. 2024 Sep 3. doi: 10.1146/annurev-ento-013124-124333. Online ahead of print. Annu Rev Entomol. 2024. PMID: 39227131 Review.
-
Endocrinology of reproduction and phase transition in locusts.Gen Comp Endocrinol. 2009 May 15;162(1):79-92. doi: 10.1016/j.ygcen.2008.11.016. Epub 2008 Dec 3. Gen Comp Endocrinol. 2009. PMID: 19084019 Review.
Cited by
-
Population Density-Dependent Developmental Regulation in Migratory Locust.Insects. 2024 Jun 11;15(6):443. doi: 10.3390/insects15060443. Insects. 2024. PMID: 38921158 Free PMC article.
-
A plant virus manipulates the long-winged morph of insect vectors.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2315341121. doi: 10.1073/pnas.2315341121. Epub 2024 Jan 8. Proc Natl Acad Sci U S A. 2024. PMID: 38190519 Free PMC article.
-
QnAs with Le Kang.Proc Natl Acad Sci U S A. 2023 May 30;120(22):e2306994120. doi: 10.1073/pnas.2306994120. Epub 2023 May 22. Proc Natl Acad Sci U S A. 2023. PMID: 37216552 Free PMC article. No abstract available.
References
-
- Wang X. H., Kang L., Molecular mechanisms of phase change in locusts. Annu. Rev. Entomol. 59, 225–244 (2014). - PubMed
-
- Pener M. P., Simpson S. J., “Locust phase polyphenism: An update” in Advances in Insect Physiology, Simpson S. J., Pener M. P., Eds. (Academic Press, 2009), Vol. 36, pp. 1–272.
-
- Ayali A., The puzzle of locust density-dependent phase polyphenism. Curr. Opin. Insect Sci. 35, 41–47 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

