CB2R agonist GW405833 alleviates acute liver failure in mice via inhibiting HIF-1α-mediated reprogramming of glycometabolism and macrophage proliferation
- PMID: 36697976
- PMCID: PMC10310807
- DOI: 10.1038/s41401-022-01037-8
CB2R agonist GW405833 alleviates acute liver failure in mice via inhibiting HIF-1α-mediated reprogramming of glycometabolism and macrophage proliferation
Abstract
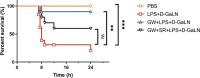
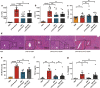
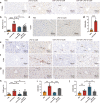
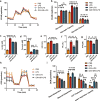
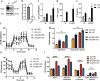
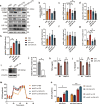
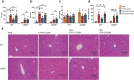
The inflammatory responses involving infiltration and activation of liver macrophages play a vital role in acute liver failure (ALF). In the liver of ALF mice, cannabinoid receptor 2 (CB2R) is significantly upregulated on macrophages, while CB2R agonist GW405833 (GW) could protect against cell death in acute liver damage. In this study, we investigated the molecular mechanisms underlying the protective effects of GW against ALF in vivo and in vitro from a perspective of macrophage glycometabolism. Mice were pretreated with GW (10 mg/kg, i.p.), then were injected with D-GalN (750 mg/kg, i.p.) and LPS (10 mg/kg, i.p.) to induce ALF. We verified the protective effects of GW pretreatment in ALF mice. Furthermore, GW pretreatment significantly reduced liver macrophage infiltration and M1 polarization, and inhibited the release of inflammatory factors TNF-α and IL-1β in ALF mice. These protective effects were eliminated by CB2R antagonist SR144528 or in CB2R-/- ALF mice. We used LPS-stimulated RAW264.7 cells as an in vitro M1 macrophage-centered model of inflammatory response, and demonstrated that pretreatment with GW (10 μM) significantly reduced glucose metabolism by inhibiting glycolysis, which inhibited LPS-induced macrophage proliferation and inflammatory cytokines release. We verified these results in a stable CB2R-/- RAW264.7 cell line. Moreover, we found that GW significantly inhibited the expression of hypoxia inducible factor 1α (HIF-1α). Using a stable HIF-1α-/- RAW264.7 cell line, we confirmed that GW reduced the release of inflammatory cytokines from macrophages and inhibited glycolysis by downregulating HIF-1α expression. In conclusion, activation of CB2Rs inhibits the proliferation of hepatic macrophages and release of inflammatory factors in ALF mice through downregulating HIF-1α to inhibit glycolysis.
Keywords: GW405833; RAW264.7 cells; SR144528; acute liver failure; cannabinoid receptor 2; glycolysis; hypoxia-inducible factor 1α; macrophages.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures
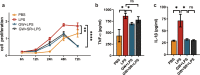
Similar articles
-
HIF-1α inhibition in macrophages preserves acute liver failure by reducing IL-1β production.FASEB J. 2023 Sep;37(9):e23140. doi: 10.1096/fj.202300428RR. FASEB J. 2023. PMID: 37584647
-
Melittin ameliorates inflammation in mouse acute liver failure via inhibition of PKM2-mediated Warburg effect.Acta Pharmacol Sin. 2021 Aug;42(8):1256-1266. doi: 10.1038/s41401-020-00516-0. Epub 2020 Sep 16. Acta Pharmacol Sin. 2021. PMID: 32939034 Free PMC article.
-
Geraniol ameliorates acute liver failure induced by lipopolysaccharide/D-galactosamine via regulating macrophage polarization and NLRP3 inflammasome activation by PPAR-γ methylation Geraniol alleviates acute liver failure.Biochem Pharmacol. 2023 Apr;210:115467. doi: 10.1016/j.bcp.2023.115467. Epub 2023 Feb 26. Biochem Pharmacol. 2023. PMID: 36849063
-
Protective effects of specific cannabinoid receptor 2 agonist GW405833 on concanavalin A-induced acute liver injury in mice.Acta Pharmacol Sin. 2019 Nov;40(11):1404-1411. doi: 10.1038/s41401-019-0213-0. Epub 2019 Mar 27. Acta Pharmacol Sin. 2019. PMID: 30918343 Free PMC article.
-
N-phenethyl-5-phenylpicolinamide alleviates inflammation in acute lung injury by inhibiting HIF-1α/glycolysis/ASIC1a pathway.Life Sci. 2022 Nov 15;309:120987. doi: 10.1016/j.lfs.2022.120987. Epub 2022 Sep 22. Life Sci. 2022. PMID: 36155179
Cited by
-
Inhibition of Expression of the Circadian Clock Gene Cryptochrome 1 Causes Abnormal Glucometabolic and Cell Growth in Bombyx mori Cells.Int J Mol Sci. 2023 Mar 12;24(6):5435. doi: 10.3390/ijms24065435. Int J Mol Sci. 2023. PMID: 36982509 Free PMC article.
-
Measuring and modeling macrophage proliferation in a lab-on-CMOS capacitance sensing microsystem.Front Bioeng Biotechnol. 2023 May 12;11:1159004. doi: 10.3389/fbioe.2023.1159004. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37251577 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

