SARS-CoV-2 neutralizing antibody therapies: an early retrospective cohort study of 26 hospitalized patients treated with bamlanivimab or casirivimab/imdevimab
- PMID: 36690138
- PMCID: PMC9859643
- DOI: 10.1016/j.ijid.2023.01.012
SARS-CoV-2 neutralizing antibody therapies: an early retrospective cohort study of 26 hospitalized patients treated with bamlanivimab or casirivimab/imdevimab
Abstract
Objectives: In this early retrospective cohort study, a total of 26 patients with SARS-CoV-2 were treated with bamlanivimab or casirivimab/imdevimab, and the reduction of the viral load associated with the developed clinical symptoms was analyzed.
Methods: Patients in the intervention groups received bamlanivimab or casirivimab/imdevimab. Patients without treatment served as control. Outcomes were assessed by clinical symptoms and change in log viral load from baseline based on the cycle threshold over a period of 18 days.
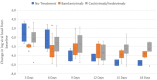
Results: Median log viral load decline was higher in both intervention groups after 3 and 6 days compared to control. However, at later time points, the decline of the viral load was more distinct in the control group. Mild symptoms of COVID-19 were observed in 6.3% of the intervention groups and in no patient of the control. No patients treated with bamlanivimab, 18.8% treated with casirivimab/imdevimab, and 14.2% in the control group developed moderate symptoms. Severe symptoms were recorded only in the control group (14.2%), including one related death.
Conclusion: Treatment with monoclonal SARS-CoV-2 antibodies seems to accelerate decline of virus loads, especially in the first 6 days after administration, compared to control. This may be associated with a reduced likeliness of a severe course of COVID-19.
Keywords: Antibody therapy; Bamlanivimab; COVID-19; Casirivimab; Imdevimab; Viral load.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no competing interests to declare.
Figures


Similar articles
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article. Review.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article. Review.
-
Curbing the Delta Surge: Clinical Outcomes After Treatment With Bamlanivimab-Etesevimab, Casirivimab-Imdevimab, or Sotrovimab for Mild to Moderate Coronavirus Disease 2019.Mayo Clin Proc. 2022 Sep;97(9):1641-1648. doi: 10.1016/j.mayocp.2022.06.015. Epub 2022 Jun 23. Mayo Clin Proc. 2022. PMID: 36058578 Free PMC article.
-
Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study.Eur J Clin Microbiol Infect Dis. 2022 Jul;41(7):1065-1076. doi: 10.1007/s10096-022-04464-x. Epub 2022 Jun 21. Eur J Clin Microbiol Infect Dis. 2022. PMID: 35727429 Free PMC article.
-
A Comparison of SARS-COV-2 Neutralizing Antibody Therapies in High-Risk Patients with Mild to Moderate COVID-19 Disease at a Single Academic Hospital.J Emerg Med. 2022 Jan;62(1):83-91. doi: 10.1016/j.jemermed.2021.07.025. Epub 2021 Jul 15. J Emerg Med. 2022. PMID: 34489146 Free PMC article.
Cited by
-
Efficacy and safety of bamlanivimab in patients with COVID-19: A systematic review and meta-analysis.World J Virol. 2024 Mar 25;13(1):88660. doi: 10.5501/wjv.v13.i1.88660. World J Virol. 2024. PMID: 38616851 Free PMC article.
References
-
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

