Functional characterization of 5' untranslated region (UTR) secondary RNA structures in the replication of tick-borne encephalitis virus in mammalian cells
- PMID: 36689554
- PMCID: PMC9894543
- DOI: 10.1371/journal.pntd.0011098
Functional characterization of 5' untranslated region (UTR) secondary RNA structures in the replication of tick-borne encephalitis virus in mammalian cells
Abstract
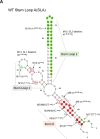
Tick-borne Encephalitis Virus (TBEV) is an emerging flavivirus that causes neurological disorders including viral encephalitis of varying severity. Whilst secondary RNA structures within the 5' untranslated regions (UTRs) of many flaviviruses determine both virus replication and pathogenic outcomes in humans, these elements have not been systematically investigated for TBEV. In this study, we investigated the role of predicted RNA secondary elements of the first 107 nucleotides (nts) of the viral genome forming the stem-loop A (SLA). Experiments were performed in replicons and infectious TBEV system. This region comprises three distinct structures: 5' stem 0 (S0), stem-loop 1 (SL1) and stem-loop 2 (SL2). S0 was found to be essential for virus infection as mutations in the lower stem of this region significantly reduced virus replication. Point mutations in SL1 that preserved the Y-shape confirmation delayed viral RNA replication but did not abolish virus infectivity. Deletion of SL2 did not abolish infectivity but had a negligible effect on virus propagation. No correlation was observed between in vitro translation efficiency and virus infectivity, suggesting that the 5'UTR functions independently to virus translation. Together, these findings reveal distinct RNA elements within the 5'UTR that are essential for the stability and replication of viral RNA. We further identify changes in RNA folding that lead to altered TBEV infectivity and pathogenesis.
Copyright: © 2023 Upstone et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation.J Virol. 2014 Jun;88(12):6611-22. doi: 10.1128/JVI.03736-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696465 Free PMC article.
-
Mutational analysis of three predicted 5'-proximal stem-loop structures in the genome of tick-borne encephalitis virus indicates different roles in RNA replication and translation.Virology. 2011 Aug 15;417(1):79-86. doi: 10.1016/j.virol.2011.05.008. Epub 2011 Jun 8. Virology. 2011. PMID: 21645915 Free PMC article.
-
[Genetic Variability of Tick-Borne Encephalitis Virus Genome 5'-UTR from Northern Eurasia].Mol Biol (Mosk). 2021 May-Jun;55(3):431-440. doi: 10.31857/S0026898421030149. Mol Biol (Mosk). 2021. PMID: 34097678 Russian.
-
Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis.Virus Res. 2005 Aug;111(2):161-74. doi: 10.1016/j.virusres.2005.04.007. Virus Res. 2005. PMID: 15871909 Review.
-
Tick-Borne Encephalitis Virus: A Structural View.Viruses. 2018 Jun 28;10(7):350. doi: 10.3390/v10070350. Viruses. 2018. PMID: 29958443 Free PMC article. Review.
Cited by
-
Untranslated Regions of a Segmented Kindia Tick Virus Genome Are Highly Conserved and Contain Multiple Regulatory Elements for Viral Replication.Microorganisms. 2024 Jan 23;12(2):239. doi: 10.3390/microorganisms12020239. Microorganisms. 2024. PMID: 38399643 Free PMC article.
-
The myriad roles of RNA structure in the flavivirus life cycle.RNA Biol. 2024 Jan;21(1):14-30. doi: 10.1080/15476286.2024.2357857. Epub 2024 May 26. RNA Biol. 2024. PMID: 38797925 Free PMC article. Review.
-
Tick-Borne Encephalitis-Review of the Current Status.J Clin Med. 2023 Oct 18;12(20):6603. doi: 10.3390/jcm12206603. J Clin Med. 2023. PMID: 37892741 Free PMC article. Review.
References
-
- Khasnatinov MA, Ustanikova K, Frolova T V, Pogodina V V, Bochkova NG, Levina LS, et al.. Non-Hemagglutinating Flaviviruses: Molecular Mechanisms for the Emergence of New Strains via Adaptation to European Ticks. PLOS ONE. 2009;4: e7295–. Available: doi: 10.1371/journal.pone.0007295 - DOI - PMC - PubMed

