Platelet-rich plasma attenuates the severity of joint capsule fibrosis following post-traumatic joint contracture in rats
- PMID: 36686225
- PMCID: PMC9845589
- DOI: 10.3389/fbioe.2022.1078527
Platelet-rich plasma attenuates the severity of joint capsule fibrosis following post-traumatic joint contracture in rats
Abstract
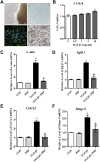
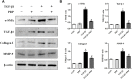
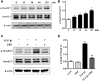
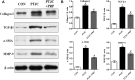
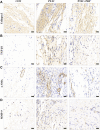
Background: Post-traumatic joint contracture (PTJC) mainly manifests as excessive inflammation leading to joint capsule fibrosis. Transforming growth factor (TGF)-β1, a key regulator of inflammation and fibrosis, can promote fibroblast activation, proliferation, migration, and differentiation into myofibroblasts. Platelet-rich plasma (PRP) is considered to have strong potential for improving tissue healing and regeneration, the ability to treat joint capsule fibrosis remains largely unknown. Methods: In this study, we aimed to determine the antifibrotic potential of PRP in vivo or in vitro and its possible molecular mechanisms. The TGF-β1-induced primary joint capsule fibroblast model and rat PTJC model were used to observe several fibrotic markers (TGF-β1, α-SMA, COL-Ⅰ, MMP-9) and signaling transduction pathway (Smad2/3) using histological staining, qRT-PCR and western blot. Results: Fibroblasts transformed to myofibroblasts after TGF-β1 stimulation with an increase of TGF-β1, α-SMA, COL-Ⅰ, MMP-9 and the activation of Smad2/3 in vitro. However, TGF-β1-induced upregulation or activation of these fibrotic markers or signaling could be effectively suppressed by the introduction of PRP. Fibrotic markers' similar changes were observed in the rat PTJC model and PRP effectively reduced inflammatory cell infiltration and collagen fiber deposition in the posterior joint capsule. Interestingly, HE staining showed that articular cartilage was degraded after rat PTJC, and PRP injection also have the potential to protect articular cartilage. Conclusion: PRP can attenuate pathological changes of joint capsule fibrosis during PTJC, which may be implemented by inhibiting TGF-β1/Smad2/3 signaling and downstream fibrotic marker expression in joint capsule fibroblasts.
Keywords: fibroblasts; joint capsule fibrosis; platelet-rich plasma; post-traumatic joint contracture; transforming growth factor-β1.
Copyright © 2023 Zhang, Wang, Zong, Gu, Fan, Xu, Cai and Lu.
Figures


Similar articles
-
Macrophage migration inhibitory factor regulates joint capsule fibrosis by promoting TGF-β1 production in fibroblasts.Int J Biol Sci. 2021 Apr 29;17(7):1837-1850. doi: 10.7150/ijbs.57025. eCollection 2021. Int J Biol Sci. 2021. PMID: 33994866 Free PMC article.
-
Macrophage migration inhibitory factor activates the inflammatory response in joint capsule fibroblasts following post-traumatic joint contracture.Aging (Albany NY). 2021 Feb 17;13(4):5804-5823. doi: 10.18632/aging.202505. Epub 2021 Feb 17. Aging (Albany NY). 2021. PMID: 33601337 Free PMC article.
-
Downregulation of interleukin 11 regulates the transforming growth factor-β/ERK1/2 signaling pathway to inhibit articular capsule fibrosis and alleviate post-traumatic articular capsule contracture.J Shoulder Elbow Surg. 2024 Jul 30:S1058-2746(24)00510-X. doi: 10.1016/j.jse.2024.05.057. Online ahead of print. J Shoulder Elbow Surg. 2024. PMID: 39089417
-
Shensong Yangxin Capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling.J Ethnopharmacol. 2014 Nov 18;157:161-70. doi: 10.1016/j.jep.2014.09.035. Epub 2014 Oct 3. J Ethnopharmacol. 2014. PMID: 25267579
-
Platelet-rich plasma affects gap junctional features in myofibroblasts in vitro via vascular endothelial growth factor (VEGF)-A/VEGF receptor.Exp Physiol. 2022 Feb;107(2):106-121. doi: 10.1113/EP090052. Epub 2022 Jan 4. Exp Physiol. 2022. PMID: 34935228
Cited by
-
The Well-Forgotten Old: Platelet-Rich Plasma in Modern Anti-Aging Therapy.Cells. 2024 Oct 23;13(21):1755. doi: 10.3390/cells13211755. Cells. 2024. PMID: 39513862 Free PMC article. Review.
-
Time-Series Expression Profile Analysis of Post-Traumatic Joint Contracture in Rats at the Early Stages of the Healing Process.J Inflamm Res. 2023 Mar 15;16:1169-1181. doi: 10.2147/JIR.S400557. eCollection 2023. J Inflamm Res. 2023. PMID: 36945316 Free PMC article.
-
Platelet Rich plasma injection of the vocal folds in benign vocal pathologies.Eur Arch Otorhinolaryngol. 2024 Oct;281(10):5419-5428. doi: 10.1007/s00405-024-08824-5. Epub 2024 Jul 17. Eur Arch Otorhinolaryngol. 2024. PMID: 39014252 Free PMC article.
References
-
- Belk J. W., Kraeutler M. J., Thon S. G., Littlefield C. P., Smith J. H., McCarty E. C. (2020). Augmentation of meniscal repair with platelet-rich plasma: A systematic review of comparative studies. Orthop. J. Sports Med. 8, 232596712092614. 10.1177/2325967120926145 PubMed Abstract | 10.1177/2325967120926145 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Bennell K. L., Hunter D. J., Paterson K. L. (2017). Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr. Rheumatol. Rep. 19, 24. 10.1007/s11926-017-0652-x PubMed Abstract | 10.1007/s11926-017-0652-x | Google Scholar - DOI - DOI - PubMed
-
- Campbell T. M., Trudel G., Wong K. K., Laneuville O. (2014). Genome wide gene expression analysis of the posterior capsule in patients with osteoarthritis and knee flexion contracture. J. Rheumatol. 41, 2232–2239. 10.3899/jrheum.140079 PubMed Abstract | 10.3899/jrheum.140079 | Google Scholar - DOI - DOI - PubMed
-
- Chellini F., Tani A., Vallone L., Nosi D., Pavan P., Bambi F., et al. (2018). Platelet-rich plasma and bone marrow-derived mesenchymal stromal cells prevent TGF-β1-induced myofibroblast generation but are not synergistic when combined: Morphological in vitro analysis. Cells Tissues Organs 206, 283–295. 10.1159/000501499 PubMed Abstract | 10.1159/000501499 | Google Scholar - DOI - DOI - PubMed
-
- Chellini F., Tani A., Vallone L., Nosi D., Pavan P., Bambi F., et al. (2018). Platelet-rich plasma prevents in vitro transforming growth factor-β1-induced fibroblast to myofibroblast transition: Involvement of vascular endothelial growth factor (VEGF)-A/VEGF receptor-1-mediated signaling †. Cells 7, 142. 10.3390/cells7090142 PubMed Abstract | 10.3390/cells7090142 | Google Scholar - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

