Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): a randomised controlled trial
- PMID: 36684392
- PMCID: PMC9852275
- DOI: 10.1016/j.eclinm.2022.101819
Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): a randomised controlled trial
Abstract
Background: 3% of kidney transplant recipients return to dialysis annually upon allograft failure. Development of antibodies (Ab) against human leukocyte antigens (HLA) is a validated prognostic biomarker of allograft failure. We tested whether screening for HLA Ab, combined with an intervention to improve adherence and optimization of immunosuppression could prevent allograft failure.
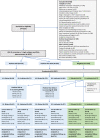
Methods: Prospective, open-labelled randomised biomarker-based strategy (hybrid) trial in 13 UK transplant centres [EudraCT (2012-004308-36) and ISRCTN (46157828)]. Patients were randomly allocated (1:1) to unblinded or double-blinded arms and screened every 8 months. Unblinded HLA Ab+ patients were interviewed to encourage medication adherence and had tailored optimisation of Tacrolimus, Mycophenolate mofetil and Prednisolone. The primary outcome was time to graft failure in an intention to treat analysis. The trial had 80% power to detect a hazard ratio of 0.49 in donor specific antibody (DSA)+ patients.
Findings: From 11/9/13 to 27/10/16, 5519 were screened for eligibility and 2037 randomised (1028 to unblinded care and 1009 to double blinded care). We identified 198 with DSA and 818 with non-DSA. Development of DSA, but not non-DSA was predictive of graft failure. HRs for graft failure in unblinded DSA+ and non-DSA+ groups were 1.54 (95% CI: 0.72 to 3.30) and 0.97 (0.54-1.74) respectively, providing no evidence of an intervention effect. Non-inferiority for the overall unblinded versus blinded comparison was not demonstrated as the upper confidence limit of the HR for graft failure exceeded 1.4 (1.02, 95% CI: 0.72 to 1.44). The only secondary endpoint reduced in the unblinded arm was biopsy-proven rejection.
Interpretation: Intervention to improve adherence and optimize immunosuppression does not delay failure of renal transplants after development of DSA. Whilst DSA predicts increased risk of allograft failure, novel interventions are needed before screening can be used to direct therapy.
Funding: The National Institute for Health Research Efficacy and Mechanism Evaluation programme grant (ref 11/100/34).
Keywords: HLA antibodies; Kidney allograft failure; Kidney transplantation; Optimised immunosuppression; Stratified medicine.
© 2023 The Authors.
Conflict of interest statement
DB declares consulting fees and speaker honoraria from Hansa Biopharma. RT declares membership of ESOT Education Committee (2018–2021) (expenses reimbursed). PM declares research funding from NIHR. AD declares research funding from the 10.13039/501100007155Medical Research Council, consulting fees (paid to KCL) from Hansa Biopharma, Verici Diagnostics, UCB Pharma and Quell Therapeutics, Membership of the Herperis Faculty 2019, 2021 and 2022 (expenses reimbursed), Membership of the UK Organ donation and transplantation research network executive since 2020 (unpaid), Membership of the EME funding Committee (2014–2019) and the EME funding committee subgroup (2018–2019) (both unpaid). The remaining authors declare no conflicts of interest.
Figures

Similar articles
-
Preventing kidney transplant failure by screening for antibodies against human leucocyte antigens followed by optimised immunosuppression: OuTSMART RCT.Southampton (UK): National Institute for Health and Care Research; 2023 Sep. Southampton (UK): National Institute for Health and Care Research; 2023 Sep. PMID: 37851847 Free Books & Documents. Review.
-
Update to the study protocol, including statistical analysis plan, for the multicentre, randomised controlled OuTSMART trial: a combined screening/treatment programme to prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies.Trials. 2019 Aug 5;20(1):476. doi: 10.1186/s13063-019-3602-2. Trials. 2019. PMID: 31383029 Free PMC article.
-
Can a combined screening/treatment programme prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies: study protocol for the multicentre randomised controlled OuTSMART trial.Trials. 2014 Jan 21;15:30. doi: 10.1186/1745-6215-15-30. Trials. 2014. PMID: 24447519 Free PMC article. Clinical Trial.
-
Development of de novo HLA donor specific antibodies (HLA-DSA), HLA antibodies (HLA-Ab) and allograft rejection post blood transfusion in kidney transplant recipients.Hum Immunol. 2020 Jul;81(7):323-329. doi: 10.1016/j.humimm.2020.04.002. Epub 2020 Apr 21. Hum Immunol. 2020. PMID: 32327243
-
The Role of Donor-Specific Antibodies in Intestinal Transplantation: Experience at the University of California Los Angeles and Literature Review.Clin Transpl. 2014:153-9. Clin Transpl. 2014. PMID: 26281140 Review.
Cited by
-
Preventing Rejection of the Kidney Transplant.J Clin Med. 2023 Sep 13;12(18):5938. doi: 10.3390/jcm12185938. J Clin Med. 2023. PMID: 37762879 Free PMC article. Review.
-
Perspective for Donor-Derived Cell-Free DNA in Antibody-Mediated Rejection After Kidney Transplantation: Defining Context of Use and Clinical Implications.Transpl Int. 2024 Aug 12;37:13239. doi: 10.3389/ti.2024.13239. eCollection 2024. Transpl Int. 2024. PMID: 39188271 Free PMC article. Review.
-
The Clinical Utility of Post-Transplant Monitoring of Donor-Specific Antibodies in Stable Renal Transplant Recipients: A Consensus Report With Guideline Statements for Clinical Practice.Transpl Int. 2023 Jul 25;36:11321. doi: 10.3389/ti.2023.11321. eCollection 2023. Transpl Int. 2023. PMID: 37560072 Free PMC article.
-
Impact of T Lymphocytes Isolated from Liver Perfusate of Deceased Brain Donors on Kidney Transplantation: Preliminary Evidence and Future Directions.J Clin Med. 2023 Jul 20;12(14):4786. doi: 10.3390/jcm12144786. J Clin Med. 2023. PMID: 37510901 Free PMC article.
-
The effect of COVID-19 vaccination on kidney function and HLA antibody formation in patients with end-stage kidney disease and on kidney replacement treatment.Clin Kidney J. 2024 Apr 25;17(5):sfae122. doi: 10.1093/ckj/sfae122. eCollection 2024 May. Clin Kidney J. 2024. PMID: 38770045 Free PMC article. No abstract available.
References
-
- Lamb K.E., Lodhi S., Meier-Kriesche H.U. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450–462. - PubMed
-
- Ravanan R., Udayaraj U., Bakran A., Steenkamp R., Williams A.J., Ansell D. Measures of care in adult renal transplant recipients in the United Kingdom (chapter 11) Nephrol Dial Transplant. 2007;22(Suppl 7):138–154. - PubMed
-
- Mizutani K., Terasaki P., Rosen A., et al. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5(9):2265–2272. - PubMed
-
- Lachmann N., Terasaki P.I., Budde K., et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87(10):1505–1513. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

