SAR study of piperidine derivatives as inhibitors of 1,4-dihydroxy-2-naphthoate isoprenyltransferase (MenA) from Mycobacterium tuberculosis
- PMID: 36682292
- PMCID: PMC9975056
- DOI: 10.1016/j.ejmech.2023.115125
SAR study of piperidine derivatives as inhibitors of 1,4-dihydroxy-2-naphthoate isoprenyltransferase (MenA) from Mycobacterium tuberculosis
Abstract
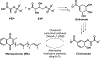
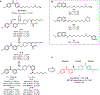
The electron transport chain (ETC) in the cell membrane consists of a series of redox complexes that transfer electrons from electron donors to acceptors and couples this electron transfer with the transfer of protons (H+) across a membrane. This process generates proton motive force which is used to produce ATP and a myriad of other functions and is essential for the long-term survival of Mycobacterium tuberculosis (Mtb), the causative organism of tuberculosis (TB), under the hypoxic conditions present within infected granulomas. Menaquinone (MK), an important carrier molecule within the mycobacterial ETC, is synthesized de novo by a cluster of enzymes known as the classic/canonical MK biosynthetic pathway. MenA (1,4-dihydroxy-2-naphthoate prenyltransferase), the antepenultimate enzyme in this pathway, is a verified target for TB therapy. In this study, we explored structure-activity relationships of a previously discovered MenA inhibitor scaffold, seeking to improve potency and drug disposition properties. Focusing our campaign upon three molecular regions, we identified two novel inhibitors with potent activity against MenA and Mtb (IC50 = 13-22 μM, GIC50 = 8-10 μM). These analogs also displayed substantially improved pharmacokinetic parameters and potent synergy with other ETC-targeting agents, achieving nearly complete sterilization of Mtb in combination therapy within two weeks in vivo. These new inhibitors of MK biosynthesis present a promising new strategy to curb the continued spread of TB.
Keywords: 1,4-dihydroxy-2-naphthoate prenyltransferase; MenA; MenA inhibitors; Menaquinone; Mtb; Mycobacterium tuberculosis; Piperidine derivatives; SAR.
Copyright © 2023 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
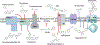
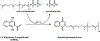




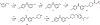
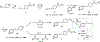
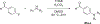
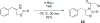
Similar articles
-
Characterization of MenA (isoprenyl diphosphate:1,4-dihydroxy-2-naphthoate isoprenyltransferase) from Mycobacterium tuberculosis.PLoS One. 2019 Apr 12;14(4):e0214958. doi: 10.1371/journal.pone.0214958. eCollection 2019. PLoS One. 2019. PMID: 30978223 Free PMC article.
-
Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis.J Med Chem. 2012 Apr 26;55(8):3739-55. doi: 10.1021/jm201608g. Epub 2012 Apr 6. J Med Chem. 2012. PMID: 22449052 Free PMC article.
-
Novel MenA Inhibitors Are Bactericidal against Mycobacterium tuberculosis and Synergize with Electron Transport Chain Inhibitors.Antimicrob Agents Chemother. 2019 May 24;63(6):e02661-18. doi: 10.1128/AAC.02661-18. Print 2019 Jun. Antimicrob Agents Chemother. 2019. PMID: 30962346 Free PMC article.
-
Unique structural and mechanistic properties of mycobacterial F-ATP synthases: Implications for drug design.Prog Biophys Mol Biol. 2020 May;152:64-73. doi: 10.1016/j.pbiomolbio.2019.11.006. Epub 2019 Nov 16. Prog Biophys Mol Biol. 2020. PMID: 31743686 Review.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
References
-
- Koegelenberg CFN, Schoch OD, Lange C, Tuberculosis: The Past, the Present and the Future, Respiration, 100 (2021) 553–556. - PubMed
-
- King A, Tuberculosis: The Forgotten Pandemic, in: Scientist The, LabX Media Group, New York City, 2021.
-
- Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR Jr., Management of drug-resistant tuberculosis, Lancet, 394 (2019) 953–966. - PubMed
-
- Gomez JE, McKinney JD, tuberculosis persistence M, latency, and drug tolerance, Tuberculosis (Edinb), 84 (2004) 29–44. - PubMed
-
- Bigger J, Treatment Of Staphylococcal Infections With Penicillin By Intermittent Sterilisation, The Lancet, 244 (1944) 497–500.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

