Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies
- PMID: 36674935
- PMCID: PMC9860943
- DOI: 10.3390/ijms24021422
Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies
Abstract
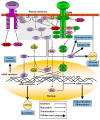
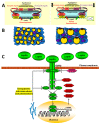
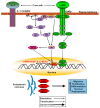
Excess body weight is frequently associated with low-grade inflammation. Evidence indicates a relationship between obesity and cancer, as well as with other diseases, such as diabetes and non-alcoholic fatty liver disease, in which inflammation and the actions of various adipokines play a role in the pathological mechanisms involved in these disorders. Leptin is mainly produced by adipose tissue in proportion to fat stores, but it is also synthesized in other organs, where leptin receptors are expressed. This hormone performs numerous actions in the brain, mainly related to the control of energy homeostasis. It is also involved in neurogenesis and neuroprotection, and central leptin resistance is related to some neurological disorders, e.g., Parkinson's and Alzheimer's diseases. In peripheral tissues, leptin is implicated in the regulation of metabolism, as well as of bone density and muscle mass. All these actions can be affected by changes in leptin levels and the mechanisms associated with resistance to this hormone. This review will present recent advances in the molecular mechanisms of leptin action and their underlying roles in pathological situations, which may be of interest for revealing new approaches for the treatment of diseases where the actions of this adipokine might be compromised.
Keywords: adipokine; biochemical mechanisms; biomarkers; cancer; inflammation; leptin; metabolic regulation; microRNA; molecular biology; obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Roles of Adipokines in Digestive Diseases: Markers of Inflammation, Metabolic Alteration and Disease Progression.Int J Mol Sci. 2020 Nov 5;21(21):8308. doi: 10.3390/ijms21218308. Int J Mol Sci. 2020. PMID: 33167521 Free PMC article. Review.
-
Association of Adipose Tissue and Adipokines with Development of Obesity-Induced Liver Cancer.Int J Mol Sci. 2021 Feb 22;22(4):2163. doi: 10.3390/ijms22042163. Int J Mol Sci. 2021. PMID: 33671547 Free PMC article. Review.
-
Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin.Proc Nutr Soc. 2012 Feb;71(1):175-80. doi: 10.1017/S0029665111003259. Epub 2011 Oct 21. Proc Nutr Soc. 2012. PMID: 22014041 Review.
-
Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease.Int J Mol Sci. 2014 Apr 11;15(4):6184-223. doi: 10.3390/ijms15046184. Int J Mol Sci. 2014. PMID: 24733068 Free PMC article. Review.
-
Molecular aspects of adipokine-bone interactions.Curr Mol Med. 2010 Aug;10(6):522-32. doi: 10.2174/1566524011009060522. Curr Mol Med. 2010. PMID: 20642443 Review.
Cited by
-
Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases.Signal Transduct Target Ther. 2024 Feb 16;9(1):37. doi: 10.1038/s41392-024-01743-1. Signal Transduct Target Ther. 2024. PMID: 38360862 Free PMC article. Review.
-
Metabolic control of puberty: 60 years in the footsteps of Kennedy and Mitra's seminal work.Nat Rev Endocrinol. 2024 Feb;20(2):111-123. doi: 10.1038/s41574-023-00919-z. Epub 2023 Dec 4. Nat Rev Endocrinol. 2024. PMID: 38049643 Free PMC article. Review.
-
Neuroprotective Effects of Leptin on the APP/PS1 Alzheimer's Disease Mouse Model: Role of Microglial and Neuroinflammation.Degener Neurol Neuromuscul Dis. 2023 Oct 25;13:69-79. doi: 10.2147/DNND.S427781. eCollection 2023. Degener Neurol Neuromuscul Dis. 2023. PMID: 37905186 Free PMC article.
-
Could Insulin Be a Better Regulator of Appetite/Satiety Balance and Body Weight Maintenance in Response to Glucose Exposure Compared to Sucrose Substitutes? Unraveling Current Knowledge and Searching for More Appropriate Choices.Med Sci (Basel). 2024 Jun 6;12(2):29. doi: 10.3390/medsci12020029. Med Sci (Basel). 2024. PMID: 38921683 Free PMC article. Review.
-
Neuroendocrinological and Clinical Aspects of Leptin.Mini Rev Med Chem. 2024;24(9):886-894. doi: 10.2174/1389557523666230825100154. Mini Rev Med Chem. 2024. PMID: 37622709 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

