Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis
- PMID: 36674855
- PMCID: PMC9864438
- DOI: 10.3390/ijms24021339
Toll-like Receptor 9 Induced Dendritic Cell Activation Promotes Anti-Myeloperoxidase Autoimmunity and Glomerulonephritis
Abstract
ANCA-associated vasculitis (AAV) is intricately linked with infections. Toll-like receptors (TLR) provide a potential link between infection and anti-myeloperoxidase (MPO) autoimmunity. TLR9 ligation has been shown to promote anti-MPO autoimmunity and glomerular vasculitis in murine MPO-AAV. This study investigates dendritic cell TLR9 ligation in murine experimental anti-MPO glomerulonephritis. We analyzed autoimmune responses to MPO following transfer of TLR9 stimulated, MPO pulsed dendritic cells and kidney injury following a sub-nephritogenic dose of sheep anti-mouse glomerular basement membrane globulin. TLR9 ligation enhanced dendritic cell activation upregulating CD40 and CD80 expression, promoting systemic anti-MPO autoimmunity and T cell recall responses and exacerbating kidney injury. CD40 upregulation by TLR9 was critical for the induction of nephritogenic autoimmunity. The presence of DEC205, which transports the TLR9 ligand to TLR9 located in the endosome, also promoted kidney injury. This confirms TLR9 mediated dendritic cell activation as a mechanism of anti-MPO autoimmunity in AAV and further defines the link between infection and the generation of MPO specific autoimmune inflammation.
Keywords: ANCA associated vasculitis; TLR9; autoimmunity; dendritic cell; glomerulonephritis; kidney injury; myeloperoxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
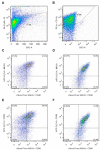
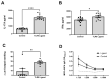
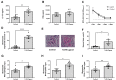

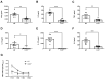

Similar articles
-
Effect of high mobility group box 1 on Toll-like receptor 9 in B cells in myeloperoxidase-ANCA-associated vasculitis.Autoimmunity. 2020 Feb;53(1):28-34. doi: 10.1080/08916934.2019.1696777. Epub 2019 Dec 2. Autoimmunity. 2020. PMID: 31790283
-
FcγRIIB regulates T-cell autoreactivity, ANCA production, and neutrophil activation to suppress anti-myeloperoxidase glomerulonephritis.Kidney Int. 2014 Dec;86(6):1140-9. doi: 10.1038/ki.2014.189. Epub 2014 May 28. Kidney Int. 2014. PMID: 24869670
-
Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis.Arthritis Rheum. 2011 Apr;63(4):1124-35. doi: 10.1002/art.30208. Arthritis Rheum. 2011. PMID: 21190299
-
Neutrophil Extracellular Traps: A Potential Therapeutic Target in MPO-ANCA Associated Vasculitis?Front Immunol. 2021 Mar 15;12:635188. doi: 10.3389/fimmu.2021.635188. eCollection 2021. Front Immunol. 2021. PMID: 33790907 Free PMC article. Review.
-
Is there a role for TNFα blockade in ANCA-associated vasculitis and glomerulonephritis?Nephrol Dial Transplant. 2017 Jan 1;32(suppl_1):i80-i88. doi: 10.1093/ndt/gfw361. Nephrol Dial Transplant. 2017. PMID: 28391344 Free PMC article. Review.
Cited by
-
Identification and validation of immune-associated NETosis subtypes and biomarkers in anti-neutrophil cytoplasmic antibody associated glomerulonephritis.Front Immunol. 2023 Jul 3;14:1177968. doi: 10.3389/fimmu.2023.1177968. eCollection 2023. Front Immunol. 2023. PMID: 37465687 Free PMC article.
-
Deciphering the Genetic Code of Autoimmune Kidney Diseases.Genes (Basel). 2023 Apr 30;14(5):1028. doi: 10.3390/genes14051028. Genes (Basel). 2023. PMID: 37239388 Free PMC article. Review.
References
-
- Wijngaarden R.A.D.L.V., Van Rijn L., Hagen E.C., Watts R.A., Gregorini G., Tervaert J.W.C., Mahr A., Niles J.L., De Heer E., Bruijn J.A., et al. Hypotheses on the Etiology of Antineutrophil Cytoplasmic Autoantibody–Associated Vasculitis: The Cause Is Hidden, but the Result Is Known. Clin. J. Am. Soc. Nephrol. 2007;3:237–252. doi: 10.2215/CJN.03550807. - DOI - PubMed
-
- McInnis E.A., Badhwar A.K., Muthigi A., Lardinois O.M., Allred S.C., Yang J., Free M.E., Jennette J.C., Preston G.A., Falk R.J., et al. Dysregulation of Autoantigen Genes in ANCA-Associated Vasculitis Involves Alternative Transcripts and New Protein Synthesis. J. Am. Soc. Nephrol. 2014;26:390–399. doi: 10.1681/ASN.2013101092. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

