Minibody-Based and scFv-Based Antibody Fragment-Drug Conjugates Selectively Eliminate GD2-Positive Tumor Cells
- PMID: 36674755
- PMCID: PMC9860947
- DOI: 10.3390/ijms24021239
Minibody-Based and scFv-Based Antibody Fragment-Drug Conjugates Selectively Eliminate GD2-Positive Tumor Cells
Abstract
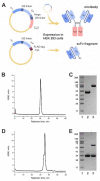
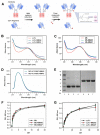
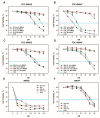
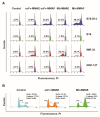
Ganglioside GD2 is a well-established target expressed on multiple solid tumors, many of which are characterized by low treatment efficiency. Antibody-drug conjugates (ADCs) have demonstrated marked success in a number of solid tumors, and GD2-directed drug conjugates may also hold strong therapeutic potential. In a recent study, we showed that ADCs based on the approved antibody dinutuximab and the drugs monomethyl auristatin E (MMAE) or F (MMAF) manifested potent and selective cytotoxicity in a panel of tumor cell lines and strongly inhibited solid tumor growth in GD2-positive mouse cancer models. Here, we employed two different GD2-binding moieties-minibodies and scFv fragments that carry variable antibody domains identical to those of dinutuximab, and site-directly conjugated them to MMAE or MMAF by thiol-maleimide chemistry with drug-to-antibody ratios (DAR) of 2 and 1, respectively. Specific binding of the antibody fragment-drug conjugates (FDCs) to GD2 was confirmed in direct ELISA, flow cytometry, and confocal microscopy. Selective cytotoxic and cytostatic effects of the conjugates were observed in GD2-positive but not GD2-negative neuroblastoma and melanoma cell lines. Minibody-based FDCs demonstrated more pronounced cytotoxic effects and stronger antigen binding compared to scFv-based FDCs. The developed molecules may offer considerable practical benefit, since antibody fragment-drug conjugates are capable of enhancing therapeutic efficacy of ADCs by improving their pharmacokinetic characteristics and reducing side effects.
Keywords: GD2-positive tumors; antibody fragments; antibody-drug conjugates; cancer; ganglioside GD2; immunotherapy; melanoma; minibodies; neuroblastoma; scFv fragments.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Targeting GD2-Positive Tumor Cells by Pegylated scFv Fragment-Drug Conjugates Carrying Maytansinoids DM1 and DM4.Curr Issues Mol Biol. 2023 Oct 5;45(10):8112-8125. doi: 10.3390/cimb45100512. Curr Issues Mol Biol. 2023. PMID: 37886955 Free PMC article.
-
Therapeutic efficacy of antibody-drug conjugates targeting GD2-positive tumors.J Immunother Cancer. 2022 Jun;10(6):e004646. doi: 10.1136/jitc-2022-004646. J Immunother Cancer. 2022. PMID: 35764367 Free PMC article.
-
Multimerization through Pegylation Improves Pharmacokinetic Properties of scFv Fragments of GD2-Specific Antibodies.Molecules. 2019 Oct 24;24(21):3835. doi: 10.3390/molecules24213835. Molecules. 2019. PMID: 31653037 Free PMC article.
-
Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy.Front Oncol. 2020 Jul 7;10:1000. doi: 10.3389/fonc.2020.01000. eCollection 2020. Front Oncol. 2020. PMID: 32733795 Free PMC article. Review.
-
Disialoganglioside GD2 as a therapeutic target for human diseases.Expert Opin Ther Targets. 2015 Mar;19(3):349-62. doi: 10.1517/14728222.2014.986459. Epub 2015 Jan 20. Expert Opin Ther Targets. 2015. PMID: 25604432 Review.
Cited by
-
Targeting GD2-Positive Tumor Cells by Pegylated scFv Fragment-Drug Conjugates Carrying Maytansinoids DM1 and DM4.Curr Issues Mol Biol. 2023 Oct 5;45(10):8112-8125. doi: 10.3390/cimb45100512. Curr Issues Mol Biol. 2023. PMID: 37886955 Free PMC article.
-
Aminooxy Click Modification of a Periodate-Oxidized Immunoglobulin G: A General Approach to Antibody-Drug Conjugates with Dye-Mediated Expeditious Stoichiometry Control.Int J Mol Sci. 2023 Mar 7;24(6):5134. doi: 10.3390/ijms24065134. Int J Mol Sci. 2023. PMID: 36982208 Free PMC article.
-
Reforming solid tumor treatment: the emerging potential of smaller format antibody-drug conjugate.Antib Ther. 2024 Feb 16;7(2):114-122. doi: 10.1093/abt/tbae005. eCollection 2024 Apr. Antib Ther. 2024. PMID: 38566971 Free PMC article. Review.
-
Penetration of Nanobody-Dextran Polymer Conjugates through Tumor Spheroids.Pharmaceutics. 2023 Sep 22;15(10):2374. doi: 10.3390/pharmaceutics15102374. Pharmaceutics. 2023. PMID: 37896133 Free PMC article.
-
Engineered Antibodies to Improve Efficacy against Neurodegenerative Disorders.Int J Mol Sci. 2024 Jun 18;25(12):6683. doi: 10.3390/ijms25126683. Int J Mol Sci. 2024. PMID: 38928395 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

