Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals
- PMID: 36674520
- PMCID: PMC9865556
- DOI: 10.3390/ijms24021008
Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals
Abstract
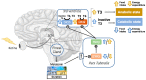
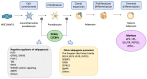
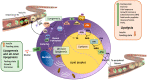
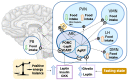
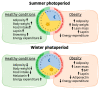
Energy homeostasis and metabolism in mammals are strongly influenced by seasonal changes. Variations in photoperiod patterns drive adaptations in body weight and adiposity, reflecting changes in the regulation of food intake and energy expenditure. Humans also show distinct patterns of energy balance depending on the season, being more susceptible to gaining weight during a specific time of the year. Changes in body weight are mainly reflected by the adipose tissue, which is a key metabolic tissue and is highly affected by circannual rhythms. Mostly, in summer-like (long-active) photoperiod, adipocytes adopt a rather anabolic profile, more predisposed to store energy, while food intake increases and energy expenditure is reduced. These metabolic adaptations involve molecular modifications, some of which have been studied during the last years and are summarized in this review. In addition, there is a bidirectional relation between obesity and the seasonal responses, with obesity disrupting some of the seasonal responses observed in healthy mammals, and altered seasonality being highly associated with increased risk of developing obesity. This suggests that changes in photoperiod produce important metabolic alterations in healthy organisms. Biological rhythms impact the regulation of metabolism to different extents, some of which are already known, but further research is needed to fully understand the relationship between energy balance and seasonality.
Keywords: adipose tissue; leptin; melatonin; obesity; photoperiod; seasonality.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish the results.
Figures
Similar articles
-
Photoperiodic programming of body weight through the neuroendocrine hypothalamus.J Endocrinol. 2003 Apr;177(1):27-34. doi: 10.1677/joe.0.1770027. J Endocrinol. 2003. PMID: 12697034 Review.
-
Photoperiod regulates lean mass accretion, but not adiposity, in growing F344 rats fed a high fat diet.PLoS One. 2015 Mar 19;10(3):e0119763. doi: 10.1371/journal.pone.0119763. eCollection 2015. PLoS One. 2015. PMID: 25789758 Free PMC article.
-
What can we learn from seasonal animals about the regulation of energy balance?Prog Brain Res. 2006;153:325-37. doi: 10.1016/S0079-6123(06)53019-5. Prog Brain Res. 2006. PMID: 16876584 Review.
-
On the value of seasonal mammals for identifying mechanisms underlying the control of food intake and body weight.Horm Behav. 2014 Jun;66(1):56-65. doi: 10.1016/j.yhbeh.2014.03.009. Epub 2014 Mar 27. Horm Behav. 2014. PMID: 24681216 Free PMC article. Review.
-
Leptin and seasonal mammals.J Neuroendocrinol. 2003 Apr;15(4):409-14. doi: 10.1046/j.1365-2826.2003.01007.x. J Neuroendocrinol. 2003. PMID: 12622842 Review.
Cited by
-
Female Psammomys obesus Are Protected from Circadian Disruption-Induced Glucose Intolerance, Cardiac Fibrosis and Adipocyte Dysfunction.Int J Mol Sci. 2024 Jul 1;25(13):7265. doi: 10.3390/ijms25137265. Int J Mol Sci. 2024. PMID: 39000372 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

