Impact of c-di-GMP on the Extracellular Proteome of Rhizobium etli
- PMID: 36671740
- PMCID: PMC9855851
- DOI: 10.3390/biology12010044
Impact of c-di-GMP on the Extracellular Proteome of Rhizobium etli
Abstract
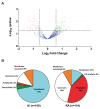
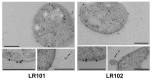
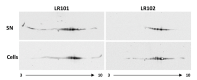
Extracellular matrix components of bacterial biofilms include biopolymers such as polysaccharides, nucleic acids and proteins. Similar to polysaccharides, the secretion of adhesins and other matrix proteins can be regulated by the second messenger cyclic diguanylate (cdG). We have performed quantitative proteomics to determine the extracellular protein contents of a Rhizobium etli strain expressing high cdG intracellular levels. cdG promoted the exportation of proteins that likely participate in adhesion and biofilm formation: the rhizobial adhesion protein RapA and two previously undescribed likely adhesins, along with flagellins. Unexpectedly, cdG also promoted the selective exportation of cytoplasmic proteins. Nearly 50% of these cytoplasmic proteins have been previously described as moonlighting or candidate moonlighting proteins in other organisms, often found extracellularly. Western blot assays confirmed cdG-promoted export of two of these cytoplasmic proteins, the translation elongation factor (EF-Tu) and glyceraldehyde 3-phosphate dehydrogenase (Gap). Transmission Electron Microscopy immunolabeling located the Gap protein in the cytoplasm but was also associated with cell membranes and extracellularly, indicative of an active process of exportation that would be enhanced by cdG. We also obtained evidence that cdG increases the number of extracellular Gap proteoforms, suggesting a link between cdG, the post-translational modification and the export of cytoplasmic proteins.
Keywords: adhesins; cyclic diguanylate; extracellular proteins; moonlighting proteins; protein PTM; rhizobia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The gap gene of Rhizobium etli is required for both free life and symbiosis with common beans.Microbiol Res. 2024 Jul;284:127737. doi: 10.1016/j.micres.2024.127737. Epub 2024 Apr 24. Microbiol Res. 2024. PMID: 38705080
-
Cyclic Di-GMP Regulates Multiple Cellular Functions in the Symbiotic Alphaproteobacterium Sinorhizobium meliloti.J Bacteriol. 2015 Nov 16;198(3):521-35. doi: 10.1128/JB.00795-15. Print 2016 Feb 1. J Bacteriol. 2015. PMID: 26574513 Free PMC article.
-
Genomic analysis of cyclic-di-GMP-related genes in rhizobial type strains and functional analysis in Rhizobium etli.Appl Microbiol Biotechnol. 2014 May;98(10):4589-602. doi: 10.1007/s00253-014-5722-7. Epub 2014 Apr 12. Appl Microbiol Biotechnol. 2014. PMID: 24728599
-
Regulation of Protein Secretion Systems Mediated by Cyclic Diguanylate in Plant-Interacting Bacteria.Front Microbiol. 2019 Jun 12;10:1289. doi: 10.3389/fmicb.2019.01289. eCollection 2019. Front Microbiol. 2019. PMID: 31263457 Free PMC article. Review.
-
Bacterial diguanylate cyclases: structure, function and mechanism in exopolysaccharide biofilm development.Biotechnol Adv. 2015 Jan-Feb;33(1):124-141. doi: 10.1016/j.biotechadv.2014.11.010. Epub 2014 Dec 10. Biotechnol Adv. 2015. PMID: 25499693 Review.
Cited by
-
Cyclic Diguanylate in the Wild: Roles During Plant and Animal Colonization.Annu Rev Microbiol. 2024 Nov;78(1):533-551. doi: 10.1146/annurev-micro-041522-101729. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 39270684 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

