Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials
- PMID: 36671605
- PMCID: PMC9854681
- DOI: 10.3390/bioengineering10010033
Stem Cell Therapy for Acute/Subacute Ischemic Stroke with a Focus on Intraarterial Stem Cell Transplantation: From Basic Research to Clinical Trials
Abstract
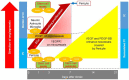
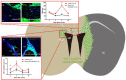
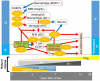
Stem cell therapy for ischemic stroke holds great promise for the treatment of neurological impairment and has moved from the laboratory into early clinical trials. The mechanism of action of stem cell therapy includes the bystander effect and cell replacement. The bystander effect plays an important role in the acute to subacute phase, and cell replacement plays an important role in the subacute to chronic phase. Intraarterial (IA) transplantation is less invasive than intraparenchymal transplantation and can provide more cells in the affected brain region than intravenous transplantation. However, transplanted cell migration was reported to be insufficient, and few transplanted cells were retained in the brain for an extended period. Therefore, the bystander effect was considered the main mechanism of action of IA stem cell transplantation. In most clinical trials, IA transplantation was performed during the acute and subacute phases. Although clinical trials of IA transplantation demonstrated safety, they did not demonstrate satisfactory efficacy in improving patient outcomes. To increase efficacy, increased migration of transplanted cells and production of long surviving and effective stem cells would be crucial. Given the lack of knowledge on this subject, we review and summarize the mechanisms of action of transplanted stem cells and recent advancements in preclinical and clinical studies to provide information and guidance for further advancement of acute/subacute phase IA stem cell transplantation therapy for ischemic stroke.
Keywords: intraarterial transplantation; ischemic stroke; regenerative medicine; stem cell transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Stem cell transplantation for ischemic stroke.Cochrane Database Syst Rev. 2019 May 5;5(5):CD007231. doi: 10.1002/14651858.CD007231.pub3. Cochrane Database Syst Rev. 2019. PMID: 31055832 Free PMC article.
-
Quantitative proteomics revealed extensive microenvironmental changes after stem cell transplantation in ischemic stroke.Front Med. 2022 Jun;16(3):429-441. doi: 10.1007/s11684-021-0842-9. Epub 2021 Jul 9. Front Med. 2022. PMID: 34241786
-
Potential Mechanisms and Perspectives in Ischemic Stroke Treatment Using Stem Cell Therapies.Front Cell Dev Biol. 2021 Apr 1;9:646927. doi: 10.3389/fcell.2021.646927. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869200 Free PMC article. Review.
-
Stem cell transplantation for ischemic stroke.Cochrane Database Syst Rev. 2010 Sep 8;(9):CD007231. doi: 10.1002/14651858.CD007231.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2019 May 05;5:CD007231. doi: 10.1002/14651858.CD007231.pub3. PMID: 20824857 Updated. Review.
-
Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): a study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke.BMC Neurol. 2017 Sep 8;17(1):179. doi: 10.1186/s12883-017-0955-6. BMC Neurol. 2017. PMID: 28886699 Free PMC article. Clinical Trial.
Cited by
-
Comparative study of the efficacy of intra-arterial and intravenous transplantation of human induced pluripotent stem cells-derived neural progenitor cells in experimental stroke.PeerJ. 2023 Nov 9;11:e16358. doi: 10.7717/peerj.16358. eCollection 2023. PeerJ. 2023. PMID: 38025691 Free PMC article.
-
Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review.Gels. 2024 Jul 18;10(7):476. doi: 10.3390/gels10070476. Gels. 2024. PMID: 39057499 Free PMC article. Review.
-
Administration of Human-Derived Mesenchymal Stem Cells Activates Locally Stimulated Endogenous Neural Progenitors and Reduces Neurological Dysfunction in Mice after Ischemic Stroke.Cells. 2024 May 29;13(11):939. doi: 10.3390/cells13110939. Cells. 2024. PMID: 38891071 Free PMC article.
-
Comparative Study of the Protective and Neurotrophic Effects of Neuronal and Glial Progenitor Cells-Derived Conditioned Media in a Model of Glutamate Toxicity In Vitro.Biomolecules. 2023 Dec 13;13(12):1784. doi: 10.3390/biom13121784. Biomolecules. 2023. PMID: 38136654 Free PMC article.
-
Stem cell-derived exosomes for ischemic stroke: a conventional and network meta-analysis based on animal models.Front Pharmacol. 2024 Oct 23;15:1481617. doi: 10.3389/fphar.2024.1481617. eCollection 2024. Front Pharmacol. 2024. PMID: 39508049 Free PMC article.
References
-
- Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/str.0000000000000211. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

