Lyophilized Progenitor Tenocyte Extracts: Sterilizable Cytotherapeutic Derivatives with Antioxidant Properties and Hyaluronan Hydrogel Functionalization Effects
- PMID: 36671025
- PMCID: PMC9854832
- DOI: 10.3390/antiox12010163
Lyophilized Progenitor Tenocyte Extracts: Sterilizable Cytotherapeutic Derivatives with Antioxidant Properties and Hyaluronan Hydrogel Functionalization Effects
Abstract
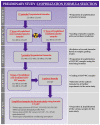
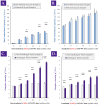
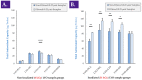
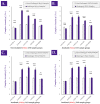
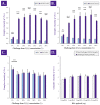
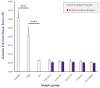
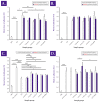
Cultured primary progenitor tenocytes in lyophilized form were previously shown to possess intrinsic antioxidant properties and hyaluronan-based hydrogel viscosity-modulating effects in vitro. The aim of this study was to prepare and functionally characterize several stabilized (lyophilized) cell-free progenitor tenocyte extracts for inclusion in cytotherapy-inspired complex injectable preparations. Fractionation and sterilization methods were included in specific biotechnological manufacturing workflows of such extracts. Comparative and functional-oriented characterizations of the various extracts were performed using several orthogonal descriptive, colorimetric, rheological, mechanical, and proteomic readouts. Specifically, an optimal sugar-based (saccharose/dextran) excipient formula was retained to produce sterilizable cytotherapeutic derivatives with appropriate functions. It was shown that extracts containing soluble cell-derived fractions possessed conserved and significant antioxidant properties (TEAC) compared to the freshly harvested cellular starting materials. Progenitor tenocyte extracts submitted to sub-micron filtration (0.22 µm) and 60Co gamma irradiation terminal sterilization (5−50 kGy) were shown to retain significant antioxidant properties and hyaluronan-based hydrogel viscosity modulating effects. Hydrogel combination products displayed important efficacy-related characteristics (friction modulation, tendon bioadhesivity) with significant (p < 0.05) protective effects of the cellular extracts in oxidative environments. Overall, the present study sets forth robust control methodologies (antioxidant assays, H2O2-challenged rheological setups) for stabilized cell-free progenitor tenocyte extracts. Importantly, it was shown that highly sensitive phases of cytotherapeutic derivative manufacturing process development (purification, terminal sterilization) allowed for the conservation of critical biological extract attributes.
Keywords: antioxidants; cell-free extracts; cytotherapies; gamma irradiation; hyaluronic acid; hydrogel viscosity; progenitor tenocytes; rheology; sterilization; tendinopathies.
Conflict of interest statement
Authors A.L., A.J. and C.P. were employed by LAM Biotechnologies SA during the course of the study. The remaining authors declare no conflicts of interest.
Figures



Similar articles
-
Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes.Gels. 2023 Oct 9;9(10):808. doi: 10.3390/gels9100808. Gels. 2023. PMID: 37888381 Free PMC article.
-
Combination of Hyaluronan and Lyophilized Progenitor Cell Derivatives: Stabilization of Functional Hydrogel Products for Therapeutic Management of Tendinous Tissue Disorders.Pharmaceutics. 2021 Dec 19;13(12):2196. doi: 10.3390/pharmaceutics13122196. Pharmaceutics. 2021. PMID: 34959477 Free PMC article.
-
Thermo-Responsive Hyaluronan-Based Hydrogels Combined with Allogeneic Cytotherapeutics for the Treatment of Osteoarthritis.Pharmaceutics. 2023 May 18;15(5):1528. doi: 10.3390/pharmaceutics15051528. Pharmaceutics. 2023. PMID: 37242774 Free PMC article.
-
Hypoxic Incubation Conditions for Optimized Manufacture of Tenocyte-Based Active Pharmaceutical Ingredients of Homologous Standardized Transplant Products in Tendon Regenerative Medicine.Cells. 2021 Oct 25;10(11):2872. doi: 10.3390/cells10112872. Cells. 2021. PMID: 34831095 Free PMC article.
-
An Effective Translation: The Development of Hyaluronan-Based Medical Products From the Physicochemical, and Preclinical Aspects.Front Bioeng Biotechnol. 2018 May 17;6:62. doi: 10.3389/fbioe.2018.00062. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 29868577 Free PMC article. Review.
Cited by
-
Bio-Enhanced Neoligaments Graft Bearing FE002 Primary Progenitor Tenocytes: Allogeneic Tissue Engineering & Surgical Proofs-of-Concept for Hand Ligament Regenerative Medicine.Pharmaceutics. 2023 Jul 3;15(7):1873. doi: 10.3390/pharmaceutics15071873. Pharmaceutics. 2023. PMID: 37514060 Free PMC article.
-
Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms.BioTech (Basel). 2023 Jan 31;12(1):15. doi: 10.3390/biotech12010015. BioTech (Basel). 2023. PMID: 36810442 Free PMC article.
-
Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes.Gels. 2023 Oct 9;9(10):808. doi: 10.3390/gels9100808. Gels. 2023. PMID: 37888381 Free PMC article.
-
Metabolomic insights into Monascus-fermented rice products: Implications for monacolin K content and nutritional optimization.Food Sci Nutr. 2024 May 13;12(8):5587-5604. doi: 10.1002/fsn3.4222. eCollection 2024 Aug. Food Sci Nutr. 2024. PMID: 39139959 Free PMC article.
-
Process Optimization and Efficacy Assessment of Standardized PRP for Tendinopathies in Sports Medicine: Retrospective Study of Clinical Files and GMP Manufacturing Records in a Swiss University Hospital.Bioengineering (Basel). 2023 Mar 25;10(4):409. doi: 10.3390/bioengineering10040409. Bioengineering (Basel). 2023. PMID: 37106596 Free PMC article.
References
-
- Laurent A., Abdel-Sayed P., Grognuz A., Scaletta C., Hirt-Burri N., Michetti M., de Buys Roessingh A.S., Raffoul W., Kronen P., Nuss K., et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines. 2021;9:380. doi: 10.3390/biomedicines9040380. - DOI - PMC - PubMed
-
- Laurent A., Porcello A., Fernandez P.G., Jeannerat A., Peneveyre C., Abdel-Sayed P., Scaletta C., Hirt-Burri N., Michetti M., de Buys Roessingh A., et al. Combination of hyaluronan and lyophilized progenitor cell derivatives: Stabilization of functional hydrogel products for therapeutic management of tendinous tissue disorders. Pharmaceutics. 2021;13:2196. doi: 10.3390/pharmaceutics13122196. - DOI - PMC - PubMed
-
- Pearce K.F., Hildebrandt M., Greinix H., Scheding S., Koehl U., Worel N., Apperley J., Edinger M., Hauser A., Mischak-Weissinger E., et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy. 2014;16:289–297. doi: 10.1016/j.jcyt.2013.08.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

