The safety and efficacy of mesenchymal stromal cells in ARDS: a meta-analysis of randomized controlled trials
- PMID: 36670442
- PMCID: PMC9857915
- DOI: 10.1186/s13054-022-04287-4
The safety and efficacy of mesenchymal stromal cells in ARDS: a meta-analysis of randomized controlled trials
Abstract
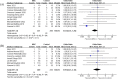
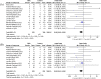
Mesenchymal stromal cells (MSC) have shown potential efficacy in both animal and human trials of acute respiratory distress syndrome (ARDS). Especially during the COVID-19 pandemic, MSC was intensely studied for treating COVID-19-induced ARDS. The purpose of this study is to evaluate the safety and efficacy of MSC in ARDS via a meta-analysis of randomized controlled trials (RCTs). Therefore, a meta-analysis of RCTs of MSC as a therapy for ARDS was conducted. The protocol of this review was registered on Open Science Framework. With no language restriction and according to the "PICOs" principle, searches were conducted on Pubmed and Embase to retrieve any clinical literature on MSC for ARDS. Any RCT, which compared MSC to controls for ARDS, where MSC and controls were intravenously infused, of any dosage, was eligible for inclusion. A total of 13 RCTs, which evaluated MSC versus control for treating ARDS, enrolling a total of 655 cases, met the inclusion criteria and appeared in this meta-analysis. A heterogeneity assessment was carried out using the χ2 test, where a P value less than 0.05 was considered significant. The choice of a fixed-effect or a random-effect model was decided by the I2 value in each of the analyses. This meta-analysis indicated that there was no significant difference in terms of adverse events between MSC and control for ARDS (OR = 0.64, 95% CI [0.34, 1.20], P = 0.17, and I2 = 0%). In comparison with control, MSC could reduce the mortality of ARDS (OR = 0.66, 95% CI [0.46, 0.96], P = 0.03, and I2 = 10%). Based on the results of our meta-analysis, the safety of MSC was demonstrated to be non-inferior to that of standard treatment, and MSC may reduce the mortality rate of ARDS. Though the heterogeneity in the main results was low (I2 < 25%), more high-quality and large-scale clinical trials are needed to further confirm our findings.
Keywords: Acute lung injury; Acute respiratory distress syndrome; Cell transplantation; Coronavirus disease 2019; Mesenchymal stromal cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 6;22(1):9. doi: 10.1186/s13063-020-04964-1. Trials. 2021. PMID: 33407777 Free PMC article.
-
Efficacy and safety of mesenchymal stem cells therapy in COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials.J Transl Med. 2024 Jun 8;22(1):550. doi: 10.1186/s12967-024-05358-6. J Transl Med. 2024. PMID: 38851730 Free PMC article. Review.
-
Safety, efficacy and biomarkers analysis of mesenchymal stromal cells therapy in ARDS: a systematic review and meta-analysis based on phase I and II RCTs.Stem Cell Res Ther. 2022 Jun 25;13(1):275. doi: 10.1186/s13287-022-02956-3. Stem Cell Res Ther. 2022. PMID: 35752865 Free PMC article. Review.
-
Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential.Expert Rev Respir Med. 2021 Mar;15(3):301-324. doi: 10.1080/17476348.2021.1848555. Epub 2020 Nov 26. Expert Rev Respir Med. 2021. PMID: 33172313 Review.
-
Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration in COVID-19 (REALIST-COVID-19): A structured summary of a study protocol for a randomised, controlled trial.Trials. 2020 Jun 3;21(1):462. doi: 10.1186/s13063-020-04416-w. Trials. 2020. PMID: 32493473 Free PMC article.
Cited by
-
Cell Therapies for Acute Radiation Syndrome.Int J Mol Sci. 2024 Jun 26;25(13):6973. doi: 10.3390/ijms25136973. Int J Mol Sci. 2024. PMID: 39000080 Free PMC article. Review.
-
Effect of mesenchymal stem cells on the host response in severe community-acquired pneumonia.Thorax. 2024 Oct 16;79(11):1086-1090. doi: 10.1136/thorax-2024-222026. Thorax. 2024. PMID: 39322407 Free PMC article. Clinical Trial.
-
The Plastic Interplay between Lung Regeneration Phenomena and Fibrotic Evolution: Current Challenges and Novel Therapeutic Perspectives.Int J Mol Sci. 2023 Dec 31;25(1):547. doi: 10.3390/ijms25010547. Int J Mol Sci. 2023. PMID: 38203718 Free PMC article. Review.
-
Enhancing regenerative medicine: the crucial role of stem cell therapy.Front Neurosci. 2024 Feb 8;18:1269577. doi: 10.3389/fnins.2024.1269577. eCollection 2024. Front Neurosci. 2024. PMID: 38389789 Free PMC article. Review.
-
MesenchymAl stromal cells for Traumatic bRain Injury (MATRIx): a study protocol for a multicenter, double-blind, randomised, placebo-controlled phase II trial.Intensive Care Med Exp. 2023 Aug 25;11(1):56. doi: 10.1186/s40635-023-00535-1. Intensive Care Med Exp. 2023. PMID: 37620640 Free PMC article.
References
-
- Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

