Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter
- PMID: 36669875
- PMCID: PMC10029822
- DOI: 10.1124/jpet.122.001454
Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter
Abstract
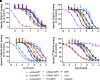
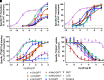
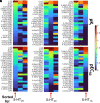
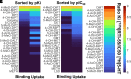
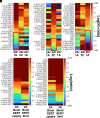
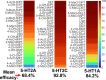
Novel psychoactive substances, including synthetic substituted tryptamines, represent a potential public health threat. Additionally, some substituted tryptamines are being studied under medical guidance as potential treatments of psychiatric disorders. Characterizing the basic pharmacology of substituted tryptamines will aid in understanding differences in potential for harm or therapeutic use. Using human embryonic kidney cells stably expressing 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT2C receptors (5-HT1AR, 5-HT2AR, and 5HT2CR, respectively) or the serotonin transporter (SERT), we measured affinities, potencies and efficacies of 21 substituted tryptamines. With the exception of two 4-acetoxy compounds, substituted tryptamines exhibited affinities and potencies less than one micromolar at the 5-HT2AR, the primary target for psychedelic effects. In comparison, half or more exhibited low affinities/potencies at 5-HT2CR, 5-HT1AR, and SERT. Sorting by the ratio of 5-HT2A to 5-HT2C, 5-HT1A, or SERT affinity revealed chemical determinants of selectivity. We found that although 4-substituted compounds exhibited affinities that ranged across a factor of 100, they largely exhibited high selectivity for 5-HT2ARs versus 5-HT1ARs and 5-HT2CRs. 5-substituted compounds exhibited high affinities for 5-HT1ARs, low affinities for 5-HT2CRs, and a range of affinities for 5-HT2ARs, resulting in selectivity for 5-HT2ARs versus 5-HT2CRs but not versus 5-HT1ARs. Additionally, a number of psychedelics bound to SERT, with non-ring-substituted tryptamines most consistently exhibiting binding. Interestingly, substituted tryptamines and known psychedelic standards exhibited a broad range of efficacies, which were lower as a class at 5-HT2ARs compared with 5-HT2CRs and 5-HT1ARs. Conversely, coupling efficiency/amplification ratio was highest at 5-HT2ARs in comparison with 5-HT2CRs and 5-HT1ARs. SIGNIFICANCE STATEMENT: Synthetic substituted tryptamines represent both potential public health threats and potential treatments of psychiatric disorders. The substituted tryptamines tested differed in affinities, potencies, and efficacies at 5-hydroxytryptamine (5-HT)2A, 5-HT2C, and 5HT1A receptors and the serotonin transporter (SERT). Several compounds were highly selective for and coupled very efficiently downstream of 5-HT2A versus 5-HT1A and 5-HT2C receptors, and some bound SERT. This basic pharmacology of substituted tryptamines helps us understand the pharmacologic basis of their potential for harm and as therapeutic agents.
U.S. Government work not protected by U.S. copyright.
Figures



Similar articles
-
Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens.Eur Neuropsychopharmacol. 2016 Aug;26(8):1327-37. doi: 10.1016/j.euroneuro.2016.05.001. Epub 2016 May 20. Eur Neuropsychopharmacol. 2016. PMID: 27216487
-
Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes.Psychopharmacology (Berl). 2014 Oct;231(21):4135-44. doi: 10.1007/s00213-014-3557-7. Epub 2014 May 7. Psychopharmacology (Berl). 2014. PMID: 24800892 Free PMC article.
-
Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors.Biochem Pharmacol. 2018 Dec;158:27-34. doi: 10.1016/j.bcp.2018.09.024. Epub 2018 Sep 25. Biochem Pharmacol. 2018. PMID: 30261175 Free PMC article.
-
"Selective" serotonin 5-HT2A receptor antagonists.Biochem Pharmacol. 2022 Jun;200:115028. doi: 10.1016/j.bcp.2022.115028. Epub 2022 Apr 4. Biochem Pharmacol. 2022. PMID: 35381208 Free PMC article. Review.
-
The Role of Central Serotonin Neurons and 5-HT Heteroreceptor Complexes in the Pathophysiology of Depression: A Historical Perspective and Future Prospects.Int J Mol Sci. 2021 Feb 15;22(4):1927. doi: 10.3390/ijms22041927. Int J Mol Sci. 2021. PMID: 33672070 Free PMC article. Review.
Cited by
-
Content analysis of Reddit posts about coadministration of selective serotonin reuptake inhibitors and psilocybin mushrooms.Psychopharmacology (Berl). 2024 Aug;241(8):1617-1630. doi: 10.1007/s00213-024-06585-x. Epub 2024 Apr 30. Psychopharmacology (Berl). 2024. PMID: 38687360
-
Drug-drug interactions involving classic psychedelics: A systematic review.J Psychopharmacol. 2024 Jan;38(1):3-18. doi: 10.1177/02698811231211219. Epub 2023 Nov 20. J Psychopharmacol. 2024. PMID: 37982394 Free PMC article. Review.
-
Pharmacologic Characterization of Substituted Nitazenes at μ, κ, and Δ Opioid Receptors Suggests High Potential for Toxicity.J Pharmacol Exp Ther. 2024 Apr 18;389(2):219-228. doi: 10.1124/jpet.123.002052. J Pharmacol Exp Ther. 2024. PMID: 38453524 Free PMC article.
-
Mechanisms and molecular targets surrounding the potential therapeutic effects of psychedelics.Mol Psychiatry. 2023 Sep;28(9):3595-3612. doi: 10.1038/s41380-023-02274-x. Epub 2023 Sep 27. Mol Psychiatry. 2023. PMID: 37759040 Free PMC article. Review.
-
Structure-activity relationships of serotonergic 5-MeO-DMT derivatives: insights into psychoactive and thermoregulatory properties.Mol Psychiatry. 2024 Aug;29(8):2346-2358. doi: 10.1038/s41380-024-02506-8. Epub 2024 Mar 14. Mol Psychiatry. 2024. PMID: 38486047 Free PMC article.
References
-
- Abbas AI, Carter A, Jeanne T, Knox R, Korthuis PT, Hamade A, Stauffer C, Uehling J (2021) Oregon Psilocybin Advisory Board Rapid Evidence Review and Recommendations, pp 1–40, Oregon Health Authority, Portland, OR.
-
- Callaway JC, Brito GS, Neves ES (2005) Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis. J Psychoactive Drugs 37:145–150. - PubMed
-
- Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, Martell J, Blemings A, Erritzoe D, Nutt DJ (2021) Trial of psilocybin versus escitalopram for depression. N Engl J Med 384:1402–1411. - PubMed
-
- Carhart-Harris RLBolstridge MRucker JDay CMErritzoe DKaelen MBloomfield MRickard JAForbes BFeilding A, et al. (2016) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3:619–627. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

