Right vagus nerve stimulation improves motor behavior by exerting neuroprotective effects in Parkinson's disease rats
- PMID: 36660708
- PMCID: PMC9843310
- DOI: 10.21037/atm-22-5366
Right vagus nerve stimulation improves motor behavior by exerting neuroprotective effects in Parkinson's disease rats
Abstract
Background: Parkinson's disease (PD) is a common movement disorder disease. Left vagus nerve stimulation (LVNS) is a potential treatment option for PD. Compared with the left vagus nerve, the right vagus nerve is more closely connected with the midbrain dopaminergic neurons, which are the lesion locations of PD. However, whether right vagus nerve stimulation (RVNS) has a therapeutic effect on PD has not yet been studied. Therefore, in this study, we studied the therapeutic effect and underlying mechanism of RVNS using a PD rat model.
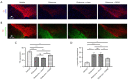
Methods: To establish the PD rat model, 8-week-old male Sprague-Dawley rats were intraperitoneally injected with rotenone for 21 days. The cuff electrodes were implanted into the right cervical vagal carotid sheaths of the rats. The right vagus nerve was continuously stimulated for 14 days using a radio stimulation system. Behavioral tests were performed before and after stimulation. Finally, tyrosine hydroxylase (TH), vesicular monoamine transporter 2 (VMAT2), and α-synuclein in the midbrain, including the substantia nigra (SN) and ventral tegmental area (VTA), were detected by immunofluorescence.
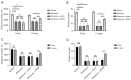
Results: A markedly lower distance traveled and rearing number was observed in the rotenone, rotenone + sham, and rotenone + RVNS groups compared to the vehicle group. After the stimulation days, the distance traveled and rearing number were both higher in the rotenone + RVNS group compared to the rotenone and rotenone + sham groups (P<0.01, P<0.0001). A remarkable increase in distance traveled and rearing number was observed in the rotenone + RVNS group after stimulation. TH expression in the vehicle group was significantly up-regulated than the other groups. RVNS markedly up-regulated TH expression level. A significantly higher expression of α-synuclein was observed in the rotenone, rotenone + sham, and rotenone + RVNS groups compared to the vehicle group. The expression of α-synuclein was lower in the rotenone + RVNS group compared to the rotenone and rotenone + sham groups. A markedly higher VMAT2 expression was observed in the vehicle group compared to other groups. RVNS significantly up-regulated VMAT2 expression.
Conclusions: The improved motor behavior and neuroprotective effects on the midbrain dopaminergic neurons in the PD rat model suggest that RVNS could be used as a potential treatment for PD.
Keywords: Parkinson’s disease (PD); rat model; right vagus nerve stimulation (RVNS); vagus nerve stimulation.
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5366/coif). TS reports funding support from the National Natural Science Foundation of China (No. 82260282). The other authors have no conflicts of interest to declare.
Figures


Similar articles
-
Left-sided vagus nerve stimulation improves cardiopulmonary resuscitation outcomes in rats as effectively as right-sided vagus nerve stimulation.World J Emerg Med. 2021;12(4):309-316. doi: 10.5847/wjem.j.1920-8642.2021.04.010. World J Emerg Med. 2021. PMID: 34512829 Free PMC article.
-
Vagus nerve stimulation with a small total charge transfer improves motor behavior and reduces neuroinflammation in a mouse model of Parkinson's disease.Neurochem Int. 2024 Nov;180:105871. doi: 10.1016/j.neuint.2024.105871. Epub 2024 Oct 1. Neurochem Int. 2024. PMID: 39362497
-
Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson's disease.Neurosci Lett. 2020 Jan 18;716:134652. doi: 10.1016/j.neulet.2019.134652. Epub 2019 Nov 25. Neurosci Lett. 2020. PMID: 31778768
-
Role of caloric vestibular stimulation in improvement of motor symptoms and inhibition of neuronal degeneration in rotenone model of Parkinson's disease - An experimental study.Physiol Int. 2020 Oct 3;107(3):390-405. doi: 10.1556/2060.2020.00036. Print 2020 Oct 17. Physiol Int. 2020. PMID: 33021954
-
Potential role of acupuncture in the treatment of Parkinson's disease: A narrative review.Integr Med Res. 2023 Jun;12(2):100954. doi: 10.1016/j.imr.2023.100954. Epub 2023 May 11. Integr Med Res. 2023. PMID: 37275921 Free PMC article. Review.
Cited by
-
Relationship Between Short-chain Fatty Acids and Parkinson's Disease: A Review from Pathology to Clinic.Neurosci Bull. 2024 Apr;40(4):500-516. doi: 10.1007/s12264-023-01123-9. Epub 2023 Sep 27. Neurosci Bull. 2024. PMID: 37755674 Free PMC article. Review.
-
Vagus nerve stimulation in Parkinson's disease: a scoping review of animal studies and human subjects research.NPJ Parkinsons Dis. 2024 Oct 24;10(1):199. doi: 10.1038/s41531-024-00803-1. NPJ Parkinsons Dis. 2024. PMID: 39448636 Free PMC article.
References
LinkOut - more resources
Full Text Sources
