Polymer-based drug delivery systems under investigation for enzyme replacement and other therapies of lysosomal storage disorders
- PMID: 36657645
- PMCID: PMC10629597
- DOI: 10.1016/j.addr.2022.114683
Polymer-based drug delivery systems under investigation for enzyme replacement and other therapies of lysosomal storage disorders
Abstract
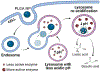
Lysosomes play a central role in cellular homeostasis and alterations in this compartment associate with many diseases. The most studied example is that of lysosomal storage disorders (LSDs), a group of 60 + maladies due to genetic mutations affecting lysosomal components, mostly enzymes. This leads to aberrant intracellular storage of macromolecules, altering normal cell function and causing multiorgan syndromes, often fatal within the first years of life. Several treatment modalities are available for a dozen LSDs, mostly consisting of enzyme replacement therapy (ERT) strategies. Yet, poor biodistribution to main targets such as the central nervous system, musculoskeletal tissue, and others, as well as generation of blocking antibodies and adverse effects hinder effective LSD treatment. Drug delivery systems are being studied to surmount these obstacles, including polymeric constructs and nanoparticles that constitute the focus of this article. We provide an overview of the formulations being tested, the diseases they aim to treat, and the results observed from respective in vitro and in vivo studies. We also discuss the advantages and disadvantages of these strategies, the remaining gaps of knowledge regarding their performance, and important items to consider for their clinical translation. Overall, polymeric nanoconstructs hold considerable promise to advance treatment for LSDs.
Keywords: Cellular and animal models; Enzyme replacement therapy; Lysosomal storage disorders; Nanoemulsions; Nanoparticles; Polymer-based drug delivery systems.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Current treatment options and novel nanotechnology-driven enzyme replacement strategies for lysosomal storage disorders.Adv Drug Deliv Rev. 2022 Sep;188:114464. doi: 10.1016/j.addr.2022.114464. Epub 2022 Jul 22. Adv Drug Deliv Rev. 2022. PMID: 35878795 Review.
-
Nanotechnology-based approaches for treating lysosomal storage disorders, a focus on Fabry disease.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 May;13(3):e1684. doi: 10.1002/wnan.1684. Epub 2020 Dec 13. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 33314628 Review.
-
Stem Cell Applications in Lysosomal Storage Disorders: Progress and Ongoing Challenges.Adv Exp Med Biol. 2021;1347:135-162. doi: 10.1007/5584_2021_639. Adv Exp Med Biol. 2021. PMID: 33977438
-
A polylysine-polyhistidine fusion peptide for lysosome-targeted protein delivery.Biochem Biophys Res Commun. 2020 Dec 17;533(4):905-912. doi: 10.1016/j.bbrc.2020.09.087. Epub 2020 Sep 30. Biochem Biophys Res Commun. 2020. PMID: 33008588
-
Advances in therapies for neurological lysosomal storage disorders.J Inherit Metab Dis. 2023 Sep;46(5):874-905. doi: 10.1002/jimd.12615. Epub 2023 May 2. J Inherit Metab Dis. 2023. PMID: 37078180 Review.
Cited by
-
Peptide-coated DNA nanostructures as a platform for control of lysosomal function in cells.Chem Eng J. 2024 Oct 15;498:155633. doi: 10.1016/j.cej.2024.155633. Epub 2024 Sep 12. Chem Eng J. 2024. PMID: 39372137
-
Fused Pyrazole Urea Analogs as Glucosylceramide Synthase Inhibitors for Treating Diseases.ACS Med Chem Lett. 2023 Jul 6;14(8):1031-1032. doi: 10.1021/acsmedchemlett.3c00271. eCollection 2023 Aug 10. ACS Med Chem Lett. 2023. PMID: 37583817 Free PMC article.
-
Role of the Lactide:Glycolide Ratio in PLGA Nanoparticle Stability and Release under Lysosomal Conditions for Enzyme Replacement Therapy of Lysosomal Storage Disorders.J Funct Biomater. 2023 Aug 25;14(9):440. doi: 10.3390/jfb14090440. J Funct Biomater. 2023. PMID: 37754854 Free PMC article.
-
Impact of mechanical cues on key cell functions and cell-nanoparticle interactions.Discov Nano. 2024 Jun 22;19(1):106. doi: 10.1186/s11671-024-04052-2. Discov Nano. 2024. PMID: 38907808 Free PMC article. Review.
-
Novel Fused Pyrazole Amide Analogs as Glucosylceramide Synthase Inhibitors for Treating Lysosomal Storage Diseases, Neurodegenerative Diseases, Cystic Diseases, and Cancer.ACS Med Chem Lett. 2023 Nov 8;14(12):1621-1622. doi: 10.1021/acsmedchemlett.3c00475. eCollection 2023 Dec 14. ACS Med Chem Lett. 2023. PMID: 38116443 Free PMC article.
References
-
- Holtzman E, Historical Fragments; Methods; Some Terminology, in: Lysosomes. Cellular Organelles Series Springer; New York, NY, 1989, pp. 1–24. 10.1007/978-1-4899-2540-4.. - DOI
-
- Seaman MNJ, Burd CG, Emr SD, Receptor signalling and the regulation of endocytic membrane transport, Curr. Opin. Cell Biol 8 (1996) 549–556, https://doi.org/10.1016/S0955-0674(96)80034-2. - DOI - PubMed

