Evaluation of the Rapid Antigen Detection Test for Diagnosing SARS-CoV-2 during the COVID-19 Pandemic: Experience from a Centralized Isolation Site in Shanghai, China
- PMID: 36655994
- PMCID: PMC9927082
- DOI: 10.1128/spectrum.04542-22
Evaluation of the Rapid Antigen Detection Test for Diagnosing SARS-CoV-2 during the COVID-19 Pandemic: Experience from a Centralized Isolation Site in Shanghai, China
Abstract
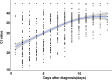
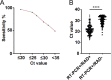
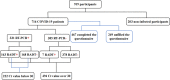
Rapid and reliable diagnosis is important for the management of individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The rapid antigen detection test (RADT) is a rapid, inexpensive, and easy method. Several studies have reported that RADTs performed well in many countries; however, very few studies have been reported in China. In this study, we assessed the performance of the RADT (Ediagnosis COVID-19 antigen test kit). This study was conducted in a centralized isolation site in Shanghai and enrolled 716 patients with COVID-19 and 203 noninfected participants. Nasopharyngeal swabs from all participants were collected on the same day and tested using the RADT and real-time reverse transcription-PCR (RT-PCR). The performance of the RADT was evaluated in different scenarios, such as threshold cycle (CT) values, symptomatic phase, and symptoms on the day of testing. The results demonstrated that the sensitivity for patients with CT values lower than 20 was 96.55% (95% confidence interval [CI], 87.05 to 99.4). The sensitivities were 78.4% (95% CI, 69.96 to 85.05) for participants within 5 days after the first RT-PCR-positive result and 90.77% (95% CI, 80.34 to 96.19) within 5 days after symptom onset. Moreover, the sensitivity of the RADT was more than 80% for patients with symptoms on the day of testing, including fever (89.29%), cough (86.84%), stuffy nose (92.59%), runny nose (92%), sore throat (81.25%), and muscle pain (80.77%), especially for those with upper respiratory tract symptoms. The specificity of the RADT was good in all scenarios. During the SARS-CoV-2 epidemic, Ediagnosis performed excellently in individuals with a higher viral load (evidenced by lower CT values), individuals in the early symptomatic phase, and especially those with upper respiratory tract symptoms. IMPORTANCE RADTs have demonstrated excellent performance in many counties for screening SARS-CoV-2 infection, but very few studies have been conducted in China. The performance of RADTs is largely related to different real-life scenarios. In our study, the performance of the RADT was evaluated in different scenarios, such as CT values, symptomatic phase, and symptoms on the day of testing. The results demonstrated that Ediagnosis (an RADT made in China) performed excellently for individuals with a higher viral load (evidenced by lower CT values), individuals in the early symptomatic phase, and especially those with upper respiratory tract symptoms.
Keywords: SARS-CoV-2; diagnostic performance; nasopharyngeal swabs; rapid antigen detection test; upper respiratory tract symptoms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A single-center experience on long-term clinical performance of a rapid SARS-CoV-2 Antigen Detection Test, STANDARD Q COVID-19 Ag Test.Sci Rep. 2023 Nov 27;13(1):20777. doi: 10.1038/s41598-023-48194-2. Sci Rep. 2023. PMID: 38012319 Free PMC article.
-
Diagnostic Performance Assessment of Saliva RT-PCR and Nasopharyngeal Antigen for the Detection of SARS-CoV-2 in Peru.Microbiol Spectr. 2022 Aug 31;10(4):e0086122. doi: 10.1128/spectrum.00861-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867471 Free PMC article.
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Molecular diagnostic approaches for SARS-CoV-2 detection and pathophysiological consequences.Mol Biol Rep. 2023 Dec;50(12):10367-10382. doi: 10.1007/s11033-023-08844-0. Epub 2023 Oct 10. Mol Biol Rep. 2023. PMID: 37817022 Review.
Cited by
-
Outcomes and risk factors of SARS-CoV-2 omicron variant in B-cell lymphoma patients following CD19 targeted CAR-T therapy.Cancer Med. 2023 Nov;12(22):20838-20846. doi: 10.1002/cam4.6657. Epub 2023 Nov 14. Cancer Med. 2023. PMID: 37962082 Free PMC article.
References
-
- Gu H, Krishnan P, Ng DYM, Chang LDJ, Liu GYZ, Cheng SSM, Hui MMY, Fan MCY, Wan JHL, Lau LHK, Cowling BJ, Peiris M, Poon LLM. 2022. Probable transmission of SARS-CoV-2 Omicron variant in quarantine hotel, Hong Kong, China, November 2021. Emerg Infect Dis 28:460–462. doi:10.3201/eid2802.212422. - DOI - PMC - PubMed
-
- The State Council Information Office, P.R.C. 19 April 2022. Press conference held on situation regarding strict prevention and control of COVID-19 epidemic. http://www.gov.cn/xinwen/gwylflkjz193/index.htm.
-
- Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, Anyaneji UJ, Bester PA, Boni MF, Chand M, Choga WT, Colquhoun R, Davids M, Deforche K, Doolabh D, Du Plessis L, Engelbrecht S, Everatt J, Giandhari J, Giovanetti M, Hardie D, Hill V, Hsiao N-Y, Iranzadeh A, Ismail A, Joseph C, Joseph R, Koopile L, Kosakovsky Pond SL, Kraemer MUG, Kuate-Lere L, Laguda-Akingba O, Lesetedi-Mafoko O, Lessells RJ, Lockman S, Lucaci AG, Maharaj A, Mahlangu B, Maponga T, Mahlakwane K, Makatini Z, Marais G, Maruapula D, Masupu K, Matshaba M, Mayaphi S, Mbhele N, Mbulawa MB, Mendes A, Mlisana K, et al. . 2022. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 603:679–686. doi:10.1038/s41586-022-04411-y. - DOI - PMC - PubMed
-
- Brandal LT, MacDonald E, Veneti L, Ravlo T, Lange H, Naseer U, Feruglio S, Bragstad K, Hungnes O, Ødeskaug LE, Hagen F, Hanch-Hansen KE, Lind A, Watle SV, Taxt AM, Johansen M, Vold L, Aavitsland P, Nygård K, Madslien EH. 2021. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill 26:2101147. doi:10.2807/1560-7917.ES.2021.26.50.2101147. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

