Mutualistic Interactions between Dinoflagellates and Pigmented Bacteria Mitigate Environmental Stress
- PMID: 36651852
- PMCID: PMC9927270
- DOI: 10.1128/spectrum.02464-22
Mutualistic Interactions between Dinoflagellates and Pigmented Bacteria Mitigate Environmental Stress
Abstract
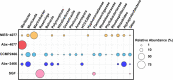
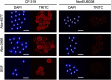
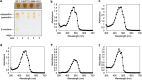
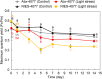
Scleractinian corals form symbiotic relationships with a variety of microorganisms, including endosymbiotic dinoflagellates of the family Symbiodiniaceae, and with bacteria, which are collectively termed coral holobionts. Interactions between hosts and their symbionts are critical to the physiological status of corals. Coral-microorganism interactions have been studied extensively, but dinoflagellate-bacterial interactions remain largely unexplored. Here, we developed a microbiome manipulation method employing KAS-antibiotic treatment (kanamycin, ampicillin, and streptomycin) to favor pigmented bacteria residing on cultured Cladocopium and Durusdinium, major endosymbionts of corals, and isolated several carotenoid-producing bacteria from cell surfaces of the microalgae. Following KAS-antibiotic treatment of Cladocopium sp. strain NIES-4077, pigmented bacteria increased 8-fold based on colony-forming assays from the parental strain, and 100% of bacterial sequences retrieved through 16S rRNA amplicon sequencing were affiliated with the genus Maribacter. Microbiome manipulation enabled host microalgae to maintain higher maximum quantum yield of photosystem II (variable fluorescence divided by maximum fluorescence [Fv/Fm]) under light-stress conditions, compared to the parental strain. Furthermore, by combining culture-dependent and -independent techniques, we demonstrated that species of the family Symbiodiniaceae and pigmented bacteria form strong interactions. Dinoflagellates protected bacteria from antibiotics, while pigmented bacteria protected microalgal cells from light stress via carotenoid production. Here, we describe for the first time a symbiotic relationship in which dinoflagellates and bacteria mutually reduce environmental stress. Investigations of microalgal-bacterial interactions further document bacterial contributions to coral holobionts and may facilitate development of novel techniques for microbiome-mediated coral reef conservation. IMPORTANCE Coral reefs cover less than 0.1% of the ocean floor, but about 25% of all marine species depend on coral reefs at some point in their life cycles. However, rising ocean temperatures associated with global climate change are a serious threat to coral reefs, causing dysfunction of the photosynthetic apparatus of endosymbiotic microalgae of corals, and overproducing reactive oxygen species harmful to corals. We manipulated the microbiome using an antibiotic treatment to favor pigmented bacteria, enabling their symbiotic microalgal partners to maintain higher photosynthetic function under insolation stress. Furthermore, we investigated mechanisms underlying microalgal-bacterial interactions, describing for the first time a symbiotic relationship in which the two symbionts mutually reduce environmental stress. Our findings extend current insights about microalgal-bacterial interactions, enabling better understanding of bacterial contributions to coral holobionts under stressful conditions and offering hope of reducing the adverse impacts of global warming on coral reefs.
Keywords: Symbiodiniaceae; carotenoid; coral holobiont; dinoflagellate; microbiome manipulation; pigmented bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A Zeaxanthin-Producing Bacterium Isolated from the Algal Phycosphere Protects Coral Endosymbionts from Environmental Stress.mBio. 2020 Jan 21;11(1):e01019-19. doi: 10.1128/mBio.01019-19. mBio. 2020. PMID: 31964724 Free PMC article.
-
Coral microbiome composition along the northern Red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities.Microbiome. 2020 Feb 3;8(1):8. doi: 10.1186/s40168-019-0776-5. Microbiome. 2020. PMID: 32008576 Free PMC article.
-
Microbiome community and complexity indicate environmental gradient acclimatisation and potential microbial interaction of endemic coral holobionts in the South China Sea.Sci Total Environ. 2021 Apr 15;765:142690. doi: 10.1016/j.scitotenv.2020.142690. Epub 2020 Oct 3. Sci Total Environ. 2021. PMID: 33071127
-
Gene clusters for biosynthesis of mycosporine-like amino acids in dinoflagellate nuclear genomes: Possible recent horizontal gene transfer between species of Symbiodiniaceae (Dinophyceae).J Phycol. 2022 Feb;58(1):1-11. doi: 10.1111/jpy.13219. Epub 2021 Nov 26. J Phycol. 2022. PMID: 34699617 Free PMC article. Review.
-
Insights into the Coral Microbiome: Underpinning the Health and Resilience of Reef Ecosystems.Annu Rev Microbiol. 2016 Sep 8;70:317-40. doi: 10.1146/annurev-micro-102215-095440. Epub 2016 Jul 8. Annu Rev Microbiol. 2016. PMID: 27482741 Review.
Cited by
-
Long-Read Sequencing Unlocks New Insights into the Amphidinium carterae Microbiome.Mar Drugs. 2024 Jul 27;22(8):342. doi: 10.3390/md22080342. Mar Drugs. 2024. PMID: 39195458 Free PMC article.
-
Astaxanthin: Past, Present, and Future.Mar Drugs. 2023 Sep 28;21(10):514. doi: 10.3390/md21100514. Mar Drugs. 2023. PMID: 37888449 Free PMC article. Review.
-
Symbiodiniaceae photophysiology and stress resilience is enhanced by microbial associations.Sci Rep. 2023 Nov 25;13(1):20724. doi: 10.1038/s41598-023-48020-9. Sci Rep. 2023. PMID: 38007500 Free PMC article.
-
Functional analyses of bacterial genomes found in Symbiodiniaceae genome assemblies.Environ Microbiol Rep. 2024 Apr;16(2):e13238. doi: 10.1111/1758-2229.13238. Environ Microbiol Rep. 2024. PMID: 38444256 Free PMC article.
-
Microalgae-bacteria nexus for environmental remediation and renewable energy resources: Advances, mechanisms and biotechnological applications.Heliyon. 2024 May 14;10(10):e31170. doi: 10.1016/j.heliyon.2024.e31170. eCollection 2024 May 30. Heliyon. 2024. PMID: 38813150 Free PMC article. Review.
References
-
- Moberg F, Folke C. 1999. Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233. doi:10.1016/S0921-8009(99)00009-9. - DOI
-
- Takagi T, Yoshioka Y, Zayasu Y, Satoh N, Shinzato C. 2020. Transcriptome analyses of immune system behaviors in primary polyp of coral Acropora digitifera exposed to the bacterial pathogen Vibrio coralliilyticus under thermal loading. Mar Biotechnol (NY) 22:748–759. doi:10.1007/s10126-020-09984-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

