Patient-specific variants of NFU1/NFU-1 disrupt cholinergic signaling in a model of multiple mitochondrial dysfunctions syndrome 1
- PMID: 36645076
- PMCID: PMC9922734
- DOI: 10.1242/dmm.049594
Patient-specific variants of NFU1/NFU-1 disrupt cholinergic signaling in a model of multiple mitochondrial dysfunctions syndrome 1
Abstract
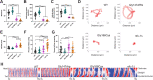
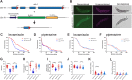
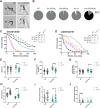
Neuromuscular dysfunction is a common feature of mitochondrial diseases and frequently presents as ataxia, spasticity and/or dystonia, all of which can severely impact individuals with mitochondrial diseases. Dystonia is one of the most common symptoms of multiple mitochondrial dysfunctions syndrome 1 (MMDS1), a disease associated with mutations in the causative gene (NFU1) that impair iron-sulfur cluster biogenesis. We have generated Caenorhabditis elegans strains that recreated patient-specific point variants in the C. elegans ortholog (nfu-1) that result in allele-specific dysfunction. Each of these mutants, Gly147Arg and Gly166Cys, have altered acetylcholine signaling at neuromuscular junctions, but opposite effects on activity and motility. We found that the Gly147Arg variant was hypersensitive to acetylcholine and that knockdown of acetylcholine release rescued nearly all neuromuscular phenotypes of this variant. In contrast, we found that the Gly166Cys variant caused predominantly postsynaptic acetylcholine hypersensitivity due to an unclear mechanism. These results are important for understanding the neuromuscular conditions of MMDS1 patients and potential avenues for therapeutic intervention.
Keywords: C. elegans; Acetylcholine; GABA; MMDS1; Mitochondria; Motility.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




Similar articles
-
Allele-specific mitochondrial stress induced by Multiple Mitochondrial Dysfunctions Syndrome 1 pathogenic mutations modeled in Caenorhabditis elegans.PLoS Genet. 2021 Aug 27;17(8):e1009771. doi: 10.1371/journal.pgen.1009771. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34449775 Free PMC article.
-
Analysis of NFU-1 metallocofactor binding-site substitutions-impacts on iron-sulfur cluster coordination and protein structure and function.FEBS J. 2017 Nov;284(22):3817-3837. doi: 10.1111/febs.14270. Epub 2017 Oct 16. FEBS J. 2017. PMID: 28906593
-
Understanding the molecular basis for multiple mitochondrial dysfunctions syndrome 1 (MMDS1): impact of a disease-causing Gly189Arg substitution on NFU1.FEBS J. 2017 Nov;284(22):3838-3848. doi: 10.1111/febs.14271. Epub 2017 Oct 12. FEBS J. 2017. PMID: 28906594 Free PMC article.
-
NFU1 gene mutation and mitochondrial disorders.Neurol India. 2016 Jul-Aug;64(4):630-2. doi: 10.4103/0028-3886.185402. Neurol India. 2016. PMID: 27381105 Review.
-
Acetylcholine.WormBook. 2007 Jan 30:1-21. doi: 10.1895/wormbook.1.131.1. WormBook. 2007. PMID: 18050502 Free PMC article. Review.
References
-
- Ahting, U., Mayr, J. A., Vanlander, A. V., Hardy, S. A., Santra, S., Makowski, C., Alston, C. L., Zimmermann, F. A., Abela, L., Plecko, B.et al. . (2015). Clinical, biochemical, and genetic spectrum of seven patients with NFU1 deficiency. Front. Genet. 6, 123. 10.3389/fgene.2015.00123 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

