Efficient Generation of Dopaminergic Neurons from Mouse Ventral Midbrain Astrocytes
- PMID: 36642416
- PMCID: PMC10129851
- DOI: 10.4062/biomolther.2022.140
Efficient Generation of Dopaminergic Neurons from Mouse Ventral Midbrain Astrocytes
Abstract
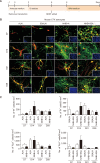
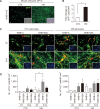
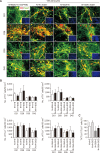
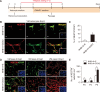
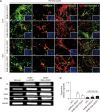
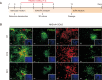
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by tremors, bradykinesia, and rigidity. PD is caused by loss of dopaminergic (DA) neurons in the midbrain substantia nigra (SN) and therefore, replenishment of DA neurons via stem cell-based therapy is a potential treatment option. Astrocytes are the most abundant non-neuronal cells in the central nervous system and are promising candidates for reprogramming into neuronal cells because they share a common origin with neurons. The ability of neural progenitor cells (NPCs) to proliferate and differentiate may overcome the limitations of the reduced viability and function of transplanted cells after cell replacement therapy. Achaete-scute complex homolog-like 1 (Ascl1) is a wellknown neuronal-specific factor that induces various cell types such as human and mouse astrocytes and fibroblasts to differentiate into neurons. Nurr1 is involved in the differentiation and maintenance of DA neurons, and decreased Nurr1 expression is known to be a major risk factor for PD. Previous studies have shown that direct conversion of astrocytes into DA neurons and NPCs can be induced by overexpression of Ascl1 and Nurr1 and additional transcription factors genes such as superoxide dismutase 1 and SRY-box 2. Here, we demonstrate that astrocytes isolated from the ventral midbrain, the origin of SN DA neurons, can be effectively converted into DA neurons and NPCs with enhanced viability. In addition, when these NPCs are inducted to differentiate, they exhibit key characteristics of DA neurons. Thus, direct conversion of midbrain astrocytes is a possible cell therapy strategy to treat neurodegenerative diseases.
Keywords: Astrocytes; Dopaminergic neurons; Transdifferentiation; Ventral midbrain.
Conflict of interest statement
There are no conflicts of interest.
Figures

Similar articles
-
ASCL1-mediated direct reprogramming: converting ventral midbrain astrocytes into dopaminergic neurons for Parkinson's disease therapy.BMB Rep. 2024 Aug;57(8):363-368. doi: 10.5483/BMBRep.2023-0222. BMB Rep. 2024. PMID: 38649147 Free PMC article.
-
Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival.Stem Cells. 2010 Mar 31;28(3):501-12. doi: 10.1002/stem.294. Stem Cells. 2010. PMID: 20049900
-
Role of Nurr1 in the Generation and Differentiation of Dopaminergic Neurons from Stem Cells.Neurotox Res. 2016 Jul;30(1):14-31. doi: 10.1007/s12640-015-9586-0. Epub 2015 Dec 17. Neurotox Res. 2016. PMID: 26678495
-
Efficient induction of dopaminergic neurons from embryonic stem cells for application to Parkinson's disease.Yonsei Med J. 2004 Jun 30;45 Suppl:23-7. doi: 10.3349/ymj.2004.45.Suppl.23. Yonsei Med J. 2004. Retraction in: Yonsei Med J. 2004 Dec 31;45(6):1203. doi: 10.3349/ymj.2004.45.6.1203 PMID: 15250046 Retracted. Review.
-
From 2D to 3D: Development of Monolayer Dopaminergic Neuronal and Midbrain Organoid Cultures for Parkinson's Disease Modeling and Regenerative Therapy.Int J Mol Sci. 2023 Jan 28;24(3):2523. doi: 10.3390/ijms24032523. Int J Mol Sci. 2023. PMID: 36768843 Free PMC article. Review.
Cited by
-
Ubiquitination of ASCL1 mediates CD47 transcriptional activation of the AKT signaling pathway, and glycolysis promotes osteogenic differentiation of hBMSCs.In Vitro Cell Dev Biol Anim. 2023 Sep;59(8):636-648. doi: 10.1007/s11626-023-00811-0. Epub 2023 Oct 2. In Vitro Cell Dev Biol Anim. 2023. PMID: 37783914 Free PMC article.
-
Recent Research Trends in Neuroinflammatory and Neurodegenerative Disorders.Cells. 2024 Mar 14;13(6):511. doi: 10.3390/cells13060511. Cells. 2024. PMID: 38534355 Free PMC article. Review.
-
ASCL1-mediated direct reprogramming: converting ventral midbrain astrocytes into dopaminergic neurons for Parkinson's disease therapy.BMB Rep. 2024 Aug;57(8):363-368. doi: 10.5483/BMBRep.2023-0222. BMB Rep. 2024. PMID: 38649147 Free PMC article.
-
The role of ferroptosis in neurodegenerative diseases.Front Cell Neurosci. 2024 Oct 15;18:1475934. doi: 10.3389/fncel.2024.1475934. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39473490 Free PMC article. Review.
References
-
- Caiazzo M., Dell'Anno M. T., Dvoretskova E., Lazarevic D., Taverna S., Leo D., Sotnikova T. D., Menegon A., Roncaglia P., Colciago G., Russo G., Carninci P., Pezzoli G., Gainetdinov R. R., Gustincich S., Dityatev A., Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. - DOI - PubMed
-
- Castro D. S., Martynoga B., Parras C., Ramesh V., Pacary E., Johnston C., Drechsel D., Lebel-Potter M., Garcia L. G., Hunt C., Dolle D., Bithell A., Ettwiller L., Buckley N., Guillemot F. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 2011;25:930–945. doi: 10.1101/gad.627811. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

