Methylenetetrahydrofolate reductase deficiency and high-dose FA supplementation disrupt embryonic development of energy balance and metabolic homeostasis in zebrafish
- PMID: 36637428
- PMCID: PMC10117162
- DOI: 10.1093/hmg/ddac308
Methylenetetrahydrofolate reductase deficiency and high-dose FA supplementation disrupt embryonic development of energy balance and metabolic homeostasis in zebrafish
Abstract
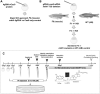
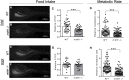
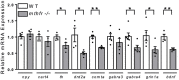
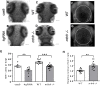
Folic acid (synthetic folate, FA) is consumed in excess in North America and may interact with common pathogenic variants in methylenetetrahydrofolate reductase (MTHFR); the most prevalent inborn error of folate metabolism with wide-ranging obesity-related comorbidities. While preclinical murine models have been valuable to inform on diet-gene interactions, a recent Folate Expert panel has encouraged validation of new animal models. In this study, we characterized a novel zebrafish model of mthfr deficiency and evaluated the effects of genetic loss of mthfr function and FA supplementation during embryonic development on energy homeostasis and metabolism. mthfr-deficient zebrafish were generated using CRISPR mutagenesis and supplemented with no FA (control, 0FA) or 100 μm FA (100FA) throughout embryonic development (0-5 days postfertilization). We show that the genetic loss of mthfr function in zebrafish recapitulates key biochemical hallmarks reported in MTHFR deficiency in humans and leads to greater lipid accumulation and aberrant cholesterol metabolism as reported in the Mthfr murine model. In mthfr-deficient zebrafish, energy homeostasis was also impaired as indicated by altered food intake, reduced metabolic rate and lower expression of central energy-regulatory genes. Microglia abundance, involved in healthy neuronal development, was also reduced. FA supplementation to control zebrafish mimicked many of the adverse effects of mthfr deficiency, some of which were also exacerbated in mthfr-deficient zebrafish. Together, these findings support the translatability of the mthfr-deficient zebrafish as a preclinical model in folate research.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice.Am J Clin Nutr. 2015 Mar;101(3):646-58. doi: 10.3945/ajcn.114.086603. Epub 2015 Jan 7. Am J Clin Nutr. 2015. PMID: 25733650 Free PMC article.
-
Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in mice.Birth Defects Res A Clin Mol Teratol. 2009 Jun;85(6):531-41. doi: 10.1002/bdra.20575. Birth Defects Res A Clin Mol Teratol. 2009. PMID: 19215022
-
Association of MTHFR 677C>T polymorphism with pregnancy outcomes in IVF/ICSI-ET recipients with adequate synthetic folic acid supplementation.Biosci Trends. 2022 Sep 17;16(4):282-290. doi: 10.5582/bst.2021.01306. Epub 2022 Jun 10. Biosci Trends. 2022. PMID: 35691911
-
[The significance of folate metabolism in complications of pregnant women].Ginekol Pol. 2013 May;84(5):377-84. doi: 10.17772/gp/1593. Ginekol Pol. 2013. PMID: 23819405 Review. Polish.
-
High Folate, Perturbed One-Carbon Metabolism and Gestational Diabetes Mellitus.Nutrients. 2022 Sep 22;14(19):3930. doi: 10.3390/nu14193930. Nutrients. 2022. PMID: 36235580 Free PMC article. Review.
Cited by
-
Targeting one-carbon metabolism for cancer immunotherapy.Clin Transl Med. 2024 Jan;14(1):e1521. doi: 10.1002/ctm2.1521. Clin Transl Med. 2024. PMID: 38279895 Free PMC article. Review.
-
Zebrafish Feed Intake: A Systematic Review for Standardizing Feeding Management in Laboratory Conditions.Biology (Basel). 2024 Mar 23;13(4):209. doi: 10.3390/biology13040209. Biology (Basel). 2024. PMID: 38666821 Free PMC article. Review.
References
-
- Scaglione, F. and Panzavolta, G. (2014) Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica, 44, 480–488. - PubMed
-
- Schwahn, B. and Rozen, R. (2001) Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences. Am. J. Pharmacogenomics, 1, 189–201. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

