Caspase-8 contributes to an immuno-hot microenvironment by promoting phagocytosis via an ecto-calreticulin-dependent mechanism
- PMID: 36635765
- PMCID: PMC9835222
- DOI: 10.1186/s40164-022-00371-1
Caspase-8 contributes to an immuno-hot microenvironment by promoting phagocytosis via an ecto-calreticulin-dependent mechanism
Abstract
Background: Caspase-8 (Casp8) acts as an initiator in cell apoptosis signaling. However, the role of Casp8 in tuning the tumor immune microenvironment remains controversial due to the complicated crosstalk between immune-tolerogenic apoptotic cell death and immunogenic cell death cascades.
Methods: The Cancer Genome Atlas (TCGA) and publicly accessible immune checkpoint blockade (ICB)-treated cohorts were used to investigate the clinical relevance of Casp8. A tumor-bearing mouse model was used to characterize changes in the tumor microenvironment and to explore the efficacy of ICB treatment under Casp8 knockout conditions.
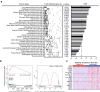
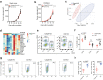
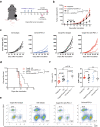
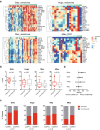
Results: By exploring TCGA datasets, we showed that the expression level of Casp8 was associated with an immuno-hot microenvironment across various solid tumor types. Casp8 deficiency leads to decreased CD8+ T cell infiltration and resistance to anti-PD-L1 therapy in a mouse model. Mechanistically, Casp8 deficiency or pharmacological disruption results in impaired ecto-calreticulin transition in tumor cells, which in turn hampers antigen presentation in draining lymph nodes. Furthermore, radiotherapy restored sensitivity to anti-PD-L1 treatment via elevated calreticulin surface expression.
Conclusions: Our data revealed a causative role of Casp8 in modulating the immunogenicity of tumor cells and responsiveness to ICB immunotherapies and proposed radiotherapy as a salvage approach to overcome Casp8 deficiency-mediated ICB resistance.
Keywords: Antigen presentation; Calreticulin; Caspase-8; Dendritic cells; Immunotherapy; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no potential competing interest.
Figures
Similar articles
-
Tumor-targeted interleukin-12 synergizes with entinostat to overcome PD-1/PD-L1 blockade-resistant tumors harboring MHC-I and APM deficiencies.J Immunother Cancer. 2022 Jun;10(6):e004561. doi: 10.1136/jitc-2022-004561. J Immunother Cancer. 2022. PMID: 35764364 Free PMC article.
-
Casp8 acts through A20 to inhibit PD-L1 expression: The mechanism and its implication in immunotherapy.Cancer Sci. 2021 Jul;112(7):2664-2678. doi: 10.1111/cas.14932. Epub 2021 May 20. Cancer Sci. 2021. PMID: 33934451 Free PMC article.
-
Low-intensity focused ultrasound targeted microbubble destruction reduces tumor blood supply and sensitizes anti-PD-L1 immunotherapy.Front Bioeng Biotechnol. 2023 Apr 17;11:1173381. doi: 10.3389/fbioe.2023.1173381. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37139047 Free PMC article.
-
Ecto-calreticulin in immunogenic chemotherapy.Immunol Rev. 2007 Dec;220:22-34. doi: 10.1111/j.1600-065X.2007.00567.x. Immunol Rev. 2007. PMID: 17979837 Review.
-
Reversing T-cell Exhaustion in Cancer: Lessons Learned from PD-1/PD-L1 Immune Checkpoint Blockade.Cancer Immunol Res. 2022 Feb;10(2):146-153. doi: 10.1158/2326-6066.CIR-21-0515. Epub 2021 Dec 22. Cancer Immunol Res. 2022. PMID: 34937730 Review.
References
-
- Kang YK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

