Hyperphosphorylated tau (p-tau) and drug discovery in the context of Alzheimer's disease and related tauopathies
- PMID: 36634842
- PMCID: PMC9975055
- DOI: 10.1016/j.drudis.2023.103487
Hyperphosphorylated tau (p-tau) and drug discovery in the context of Alzheimer's disease and related tauopathies
Abstract
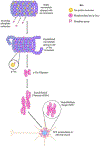
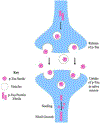
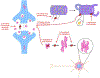
Alzheimer's disease (AD) is the most common form of dementia, characterized by intracellular neurofibrillary tangles (NFTs) and extracellular β-amyloid (βA) plaques. No disease-modifying therapy is currently available to prevent the progression of, or cure, the disease. Misfolded hyperphosphorylated tau (p-tau) is considered a pivotal point in the pathogenesis of AD and other tauopathies. Compelling evidence suggests that it is a key driver of the accumulation of NFTs and can be directly correlated with the extent of dementia in patients with AD. Therefore, inhibiting tau hyperphosphorylation-induced aggregation could be a viable strategy to discover and develop therapeutics for patients with AD.
Keywords: Alzheimer’s; amyloid; dementia; hyperphosphorylation; tau; therapy.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
None declared by authors.
Figures

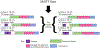
Similar articles
-
Pathological Changes of Tau Related to Alzheimer's Disease.ACS Chem Neurosci. 2019 Feb 20;10(2):931-944. doi: 10.1021/acschemneuro.8b00457. Epub 2018 Oct 23. ACS Chem Neurosci. 2019. PMID: 30346708 Review.
-
Tau-directed drug discovery for Alzheimer's disease and related tauopathies: a focus on tau assembly inhibitors.Exp Neurol. 2010 Jun;223(2):304-10. doi: 10.1016/j.expneurol.2009.08.031. Epub 2009 Sep 8. Exp Neurol. 2010. PMID: 19744482 Free PMC article. Review.
-
Novel drugs affecting tau behavior in the treatment of Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2011 Sep;8(6):678-85. doi: 10.2174/156720511796717122. Curr Alzheimer Res. 2011. PMID: 21605038 Review.
-
Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies.Biomolecules. 2016 Jan 6;6(1):6. doi: 10.3390/biom6010006. Biomolecules. 2016. PMID: 26751493 Free PMC article. Review.
-
Tau as a therapeutic target for Alzheimer's disease.Curr Alzheimer Res. 2011 Sep;8(6):666-77. doi: 10.2174/156720511796717195. Curr Alzheimer Res. 2011. PMID: 21679154 Free PMC article. Review.
Cited by
-
Hypoglycemia and Alzheimer Disease Risk: The Possible Role of Dasiglucagon.Cell Mol Neurobiol. 2024 Jul 8;44(1):55. doi: 10.1007/s10571-024-01489-y. Cell Mol Neurobiol. 2024. PMID: 38977507 Free PMC article. Review.
-
The association between rs6859 in NECTIN2 gene and Alzheimer's disease is partly mediated by pTau.medRxiv [Preprint]. 2024 Jun 22:2024.06.21.24309310. doi: 10.1101/2024.06.21.24309310. medRxiv. 2024. Update in: Front Aging Neurosci. 2024 Aug 06;16:1388363. doi: 10.3389/fnagi.2024.1388363. PMID: 38947013 Free PMC article. Updated. Preprint.
-
LncRNA ENST00000440246.1 Promotes Alzheimer's Disease Progression by Targeting PP2A.Biochem Genet. 2024 Jun;62(3):2100-2116. doi: 10.1007/s10528-023-10552-0. Epub 2023 Oct 19. Biochem Genet. 2024. PMID: 37856039
-
Mechanistic insights into MARK4 inhibition by galantamine toward therapeutic targeting of Alzheimer's disease.Front Pharmacol. 2023 Sep 19;14:1276179. doi: 10.3389/fphar.2023.1276179. eCollection 2023. Front Pharmacol. 2023. PMID: 37795023 Free PMC article.
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
References
-
- WHO. Global Action Plan on the Public Health Response to Dementia 2017–2025. Geneva; WHO: 2017.
-
- NIA. Alzheimer’s Disease Fact Sheet. www.nia.nih.gov/health/alzheimers-disease-fact-sheet [Accessed 3 January 2023].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

