Portable light-sheet optofluidic microscopy for 3D fluorescence imaging flow cytometry
- PMID: 36633262
- PMCID: PMC9931680
- DOI: 10.1039/d2lc01024k
Portable light-sheet optofluidic microscopy for 3D fluorescence imaging flow cytometry
Abstract
Imaging flow cytometry (IFC) combines conventional flow cytometry with optical microscopy, allowing for high-throughput, multi-parameter screening of single-cell specimens with morphological and spatial information. However, current 3D IFC systems are limited by instrumental complexity and incompatibility with available microfluidic devices or operations. Here, we report portable light-sheet optofluidic microscopy (PLSOM) for 3D fluorescence cytometric imaging. PLSOM exploits a compact, open-top light-sheet configuration compatible with commonly adopted microfluidic chips. The system offers a subcellular resolution (2-4 μm) in all three dimensions, high throughput (∼1000 cells per s), and portability (30 cm (l) × 10 cm (w) × 26 cm (h)). We demonstrated PLSOM for 3D IFC using various phantom and cell systems. The low-cost and custom-built architecture of PLSOM permits easy adaptability and dissemination for broad 3D flow cytometric investigations.
Conflict of interest statement
Conflicts of interest
G.A.K. is co-founder and equity shareholder of Glympse Bio and Port Therapeutics. This study could affect his personal financial status. The terms of this arrangement have been reviewed and approved by Georgia Tech in accordance with its conflict-of-interest policies.
Figures



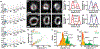
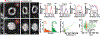
Similar articles
-
Structured-light-sheet imaging in an integrated optofluidic platform.Lab Chip. 2023 Dec 20;24(1):34-46. doi: 10.1039/d3lc00639e. Lab Chip. 2023. PMID: 37791882
-
Light-field flow cytometry for high-resolution, volumetric and multiparametric 3D single-cell analysis.Nat Commun. 2024 Mar 4;15(1):1975. doi: 10.1038/s41467-024-46250-7. Nat Commun. 2024. PMID: 38438356 Free PMC article.
-
High-throughput imaging flow cytometry by optofluidic time-stretch microscopy.Nat Protoc. 2018 Jul;13(7):1603-1631. doi: 10.1038/s41596-018-0008-7. Nat Protoc. 2018. PMID: 29976951
-
High-throughput microfluidic imaging flow cytometry.Curr Opin Biotechnol. 2019 Feb;55:36-43. doi: 10.1016/j.copbio.2018.08.002. Epub 2018 Aug 15. Curr Opin Biotechnol. 2019. PMID: 30118968 Review.
-
Three-dimensional imaging flow cytometry through light-sheet fluorescence microscopy.Cytometry A. 2017 Feb;91(2):144-151. doi: 10.1002/cyto.a.23046. Epub 2017 Jan 11. Cytometry A. 2017. PMID: 28075531 Review.
Cited by
-
A practical guide to light-sheet microscopy for nanoscale imaging: Looking beyond the cell.J Appl Phys. 2024 Sep 7;136(9):091101. doi: 10.1063/5.0218262. Epub 2024 Sep 4. J Appl Phys. 2024. PMID: 39247785 Free PMC article.
-
Frontiers in artificial intelligence-directed light-sheet microscopy for uncovering biological phenomena and multi-organ imaging.View (Beijing). 2024 Oct;5(5):20230087. doi: 10.1002/VIW.20230087. Epub 2024 Sep 3. View (Beijing). 2024. PMID: 39478956
References
-
- Huang NT, Zhang HL, Chung MT, Seo JH and Kurabayashi K, Lab Chip, 2014, 14, 1230–1245. - PubMed
-
- Psaltis D, Quake SR and Yang C, Nature, 2006, 442, 381–386. - PubMed
-
- Minzioni P, Osellame R, Sada C, Zhao S, Omenetto FG, Gylfason KB, Haraldsson T, Zhang YB, Ozcan A, Wax A, Mugele F, Schmidt H, Testa G, Bernini R, Guck J, Liberale C, Berg-Sorensen K, Chen J, Pollnau M, Xiong S, Liu AQ, Shiue CC, Fan SK, Erickson D and Sinton D, Journal of Optics, 2017, 19, 093003.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

