Gemcitabine alters sialic acid binding of the glycocalyx and induces inflammatory cytokine production in cultured endothelial cells
- PMID: 36622466
- PMCID: PMC9828377
- DOI: 10.1007/s00795-022-00347-4
Gemcitabine alters sialic acid binding of the glycocalyx and induces inflammatory cytokine production in cultured endothelial cells
Abstract
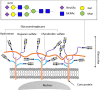
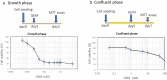
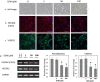
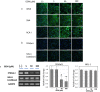
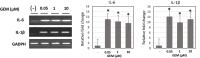
Gemcitabine (GEM) is an anticancer drug inhibiting DNA synthesis. Glomerular thrombotic microangiopathy (TMA) has been reported as an adverse effect. However, the precise mechanism of GEM-induced endothelial injury remains unknown. Cultured human umbilical vein endothelial cells (HUVECs) in the confluent phase were exposed to GEM (5-100 μM) for 48 h and evaluated cell viability and morphology, lectin binding concerning sialic acid of endothelial glycocalyx (GCX), and immunofluorescent staining of platelet-endothelial cell adhesion molecule (PECAM) and vascular endothelial growth factor receptor 2 (VEGFR2). The mRNA expression of α2,6-sialyltransferase (ST6Gal1), sialidase (neuraminidase-1: NEU-1), and interleukin (IL)-1β and IL-6 was also evaluated. GEM exposure at 5 μM induced cellular shrinkage and intercellular dissociation, accompanied by slight attenuation of PECAM and VEGFR2 immunostaining, although cell viability was still preserved. At this concentration, lectin binding showed a reduction of terminal sialic acids in endothelial GCX, probably associated with reduced ST6Gal1 mRNA expression. IL-1β and IL-6 mRNA expression was significantly increased after GEM exposure. GEM reduced terminal sialic acids in endothelial GCX through mRNA suppression of ST6Gal1 and induced inflammatory cytokine production in HUVECs. This phenomenon could be associated with the mechanism of GEM-induced TMA.
Keywords: Endothelial cell; Gemcitabine; Glycocalyx; Interleukin-1β; Interleukin-6; Platelet–endothelial cell adhesion molecule (PECAM); Sialic acid; Vascular endothelial growth factor receptor 2 (VEGFR2).
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest in any components of this study and the manuscript.
Figures
Similar articles
-
Interaction of platelet endothelial cell adhesion molecule (PECAM) with α2,6-sialylated glycan regulates its cell surface residency and anti-apoptotic role.J Biol Chem. 2014 Oct 3;289(40):27604-13. doi: 10.1074/jbc.M114.563585. Epub 2014 Aug 18. J Biol Chem. 2014. PMID: 25135639 Free PMC article.
-
Endothelial alpha 2,6-linked sialic acid inhibits VCAM-1-dependent adhesion under flow conditions.J Immunol. 1999 Sep 1;163(5):2867-76. J Immunol. 1999. PMID: 10453033
-
The Role of Sialylated Glycans in Human Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)-mediated Trans Homophilic Interactions and Endothelial Cell Barrier Function.J Biol Chem. 2016 Dec 9;291(50):26216-26225. doi: 10.1074/jbc.M116.756502. Epub 2016 Oct 28. J Biol Chem. 2016. PMID: 27793989 Free PMC article.
-
Sweet role of platelet endothelial cell adhesion molecule in understanding angiogenesis.Glycobiology. 2014 Dec;24(12):1260-4. doi: 10.1093/glycob/cwu094. Epub 2014 Sep 11. Glycobiology. 2014. PMID: 25214153 Review.
-
Endothelial Glycocalyx-Mediated Intercellular Interactions: Mechanisms and Implications for Atherosclerosis and Cancer Metastasis.Cardiovasc Eng Technol. 2021 Feb;12(1):72-90. doi: 10.1007/s13239-020-00487-7. Epub 2020 Sep 30. Cardiovasc Eng Technol. 2021. PMID: 33000443 Free PMC article. Review.
Cited by
-
Serum components influence antibody reactivity to glycan and DNA antigens.Sci Rep. 2023 Aug 22;13(1):13644. doi: 10.1038/s41598-023-40707-3. Sci Rep. 2023. PMID: 37607950 Free PMC article.
References
-
- Izzedine H, Isnard-Bagnis C, Launay-Vacher V, Mercadal L, Tostivint I, Rixe O, Brocheriou I, Bourry E, Karie S, Saeb S, Casimir N, Billemont B, Deray G. Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant. 2006;21:3038–3045. doi: 10.1093/ndt/gfl507. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

