Ribosome biogenesis in disease: new players and therapeutic targets
- PMID: 36617563
- PMCID: PMC9826790
- DOI: 10.1038/s41392-022-01285-4
Ribosome biogenesis in disease: new players and therapeutic targets
Abstract
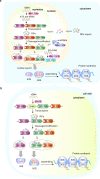
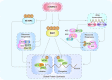
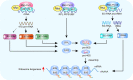
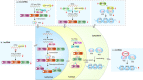
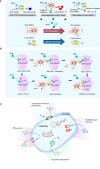
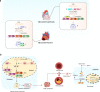
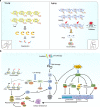
The ribosome is a multi-unit complex that translates mRNA into protein. Ribosome biogenesis is the process that generates ribosomes and plays an essential role in cell proliferation, differentiation, apoptosis, development, and transformation. The mTORC1, Myc, and noncoding RNA signaling pathways are the primary mediators that work jointly with RNA polymerases and ribosome proteins to control ribosome biogenesis and protein synthesis. Activation of mTORC1 is required for normal fetal growth and development and tissue regeneration after birth. Myc is implicated in cancer development by enhancing RNA Pol II activity, leading to uncontrolled cancer cell growth. The deregulation of noncoding RNAs such as microRNAs, long noncoding RNAs, and circular RNAs is involved in developing blood, neurodegenerative diseases, and atherosclerosis. We review the similarities and differences between eukaryotic and bacterial ribosomes and the molecular mechanism of ribosome-targeting antibiotics and bacterial resistance. We also review the most recent findings of ribosome dysfunction in COVID-19 and other conditions and discuss the consequences of ribosome frameshifting, ribosome-stalling, and ribosome-collision. We summarize the role of ribosome biogenesis in the development of various diseases. Furthermore, we review the current clinical trials, prospective vaccines for COVID-19, and therapies targeting ribosome biogenesis in cancer, cardiovascular disease, aging, and neurodegenerative disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
c-Myc overexpression increases ribosome biogenesis and protein synthesis independent of mTORC1 activation in mouse skeletal muscle.Am J Physiol Endocrinol Metab. 2021 Oct 1;321(4):E551-E559. doi: 10.1152/ajpendo.00164.2021. Epub 2021 Aug 23. Am J Physiol Endocrinol Metab. 2021. PMID: 34423683
-
mTORC1 signaling controls multiple steps in ribosome biogenesis.Semin Cell Dev Biol. 2014 Dec;36:113-20. doi: 10.1016/j.semcdb.2014.08.004. Epub 2014 Aug 19. Semin Cell Dev Biol. 2014. PMID: 25148809 Review.
-
Ribosome Biogenesis: A Central Player in Cancer Metastasis and Therapeutic Resistance.Cancer Res. 2022 Jul 5;82(13):2344-2353. doi: 10.1158/0008-5472.CAN-21-4087. Cancer Res. 2022. PMID: 35303060 Free PMC article. Review.
-
AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer.Sci Signal. 2011 Aug 30;4(188):ra56. doi: 10.1126/scisignal.2001754. Sci Signal. 2011. PMID: 21878679
-
Inhibition of mTORC1 differentially affects ribosome biogenesis in rat soleus muscle at the early and later stages of hindlimb unloading.Arch Biochem Biophys. 2022 Nov 15;730:109411. doi: 10.1016/j.abb.2022.109411. Epub 2022 Sep 22. Arch Biochem Biophys. 2022. PMID: 36155780
Cited by
-
Targeting Translation and the Cell Cycle Inversely Affects CTC Metabolism but Not Metastasis.Cancers (Basel). 2023 Nov 2;15(21):5263. doi: 10.3390/cancers15215263. Cancers (Basel). 2023. PMID: 37958436 Free PMC article.
-
The Nucleolus and Its Interactions with Viral Proteins Required for Successful Infection.Cells. 2024 Sep 21;13(18):1591. doi: 10.3390/cells13181591. Cells. 2024. PMID: 39329772 Free PMC article. Review.
-
Transient Adaptation of Toxoplasma gondii to Exposure by Thiosemicarbazone Drugs That Target Ribosomal Proteins Is Associated with the Upregulated Expression of Tachyzoite Transmembrane Proteins and Transporters.Int J Mol Sci. 2024 Aug 21;25(16):9067. doi: 10.3390/ijms25169067. Int J Mol Sci. 2024. PMID: 39201756 Free PMC article.
-
Tissue-specific O-GlcNAcylation profiling identifies substrates in translational machinery in Drosophila mushroom body contributing to olfactory learning.Elife. 2024 Apr 15;13:e91269. doi: 10.7554/eLife.91269. Elife. 2024. PMID: 38619103 Free PMC article.
-
Comprehensive analysis to identify PUS7 as a prognostic biomarker from pan-cancer analysis to osteosarcoma validation.Aging (Albany NY). 2024 May 30;16(10):9188-9203. doi: 10.18632/aging.205863. Epub 2024 May 30. Aging (Albany NY). 2024. PMID: 38819212 Free PMC article.
References
-
- Fan Y, et al. Phosphoproteomic analysis of neonatal regenerative myocardium revealed important roles of checkpoint kinase 1 via activating mammalian target of rapamycin C1/ribosomal protein S6 kinase b-1 pathway. Circulation. 2020;141:1554–1569. doi: 10.1161/CIRCULATIONAHA.119.040747. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

