Objective response rate targets for recurrent glioblastoma clinical trials based on the historic association between objective response rate and median overall survival
- PMID: 36617262
- PMCID: PMC10237425
- DOI: 10.1093/neuonc/noad002
Objective response rate targets for recurrent glioblastoma clinical trials based on the historic association between objective response rate and median overall survival
Abstract
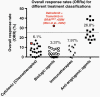
Durable objective response rate (ORR) remains a meaningful endpoint in recurrent cancer; however, the target ORR for single-arm recurrent glioblastoma trials has not been based on historic information or tied to patient outcomes. The current study reviewed 68 treatment arms comprising 4793 patients in past trials in recurrent glioblastoma in order to judiciously define target ORRs for use in recurrent glioblastoma trials. ORR was estimated at 6.1% [95% CI 4.23; 8.76%] for cytotoxic chemothera + pies (ORR = 7.59% for lomustine, 7.57% for temozolomide, 0.64% for irinotecan, and 5.32% for other agents), 3.37% for biologic agents, 7.97% for (select) immunotherapies, and 26.8% for anti-angiogenic agents. ORRs were significantly correlated with median overall survival (mOS) across chemotherapy (R2= 0.4078, P < .0001), biologics (R2= 0.4003, P = .0003), and immunotherapy trials (R2= 0.8994, P < .0001), but not anti-angiogenic agents (R2= 0, P = .8937). Pooling data from chemotherapy, biologics, and immunotherapy trials, a meta-analysis indicated a strong correlation between ORR and mOS (R2= 0.3900, P < .0001; mOS [weeks] = 1.4xORR + 24.8). Assuming an ineffective cytotoxic (control) therapy has ORR = 7.6%, the average ORR for lomustine and temozolomide trials, a sample size of ≥40 patients with target ORR>25% is needed to demonstrate statistical significance compared to control with a high level of confidence (P < .01) and adequate power (>80%). Given this historic data and potential biases in patient selection, we recommend that well-controlled, single-arm phase II studies in recurrent glioblastoma should have a target ORR >25% (which translates to a median OS of approximately 15 months) and a sample size of ≥40 patients, in order to convincingly demonstrate antitumor activity. Crucially, this response needs to have sufficient durability, which was not addressed in the current study.
Keywords: glioblastoma; objective response rate; overall survival; recurrent GBM.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
BME is a Paid Consultant and Advisor for Siemens; Medicenna; MedQIA; Imaging Endpoints; Agios Pharmaceuticals; Neosoma; Janssen; Kazia; VBL; Oncoceutics; Boston Biomedical Inc; ImmunoGenesis; and Ellipses Pharma. BME has received grant funding from Siemens, Agios, and Janssen. T.F.C. is cofounder, major stock holder, consultant and board member of Katmai Pharmaceuticals, member of the board for the 501c3 Global Coalition for Adaptive Research, holds stock option of Notable Labs, holds stock in Chimerix and receives milestone payments and possible future royalties, member of the scientific advisory board for Break Through Cancer, member of the scientific advisory board for Cure Brain Cancer Foundation, has provided paid consulting services to GCAR; Gan & Lee; BrainStorm; Katmai; Sapience; Inovio; Vigeo Therapeutics; DNATrix; Tyme; SDP; Novartis; Roche; Kintara; Bayer; Merck; Boehinger Ingelheim; VBL; Amgen; Kiyatec; Odonate Therapeutics QED; Medefield; Pascal Biosciences; Bayer; Tocagen; Karyopharm; GW Pharma; Abbvie; VBI; Deciphera; VBL; Agios; Genocea; Celgene; Puma; Lilly; BMS; Cortice; Wellcome Trust; Novocure; Novogen; Boston Biomedical; Sunovion; Human Longevity; Insys; ProNai; Pfizer; Notable labs; Medqia Trizel; Medscape and has contracts with UCLA for the Brain Tumor Program with Oncovir; Merck; Oncoceutics; Novartis; Amgen; Abbvie; DNAtrix; Beigene; BMS; AstraZeneca; Kazia; Agios; Boston Biomedical; Deciphera; Tocagen; Orbus; AstraZenica; Karyopharm. M.J.v.d.B. has received honoraria from Carthera; Genenta; Nerviano; Cellgene; Astra Zeneca and Boehringer. H.C. has received in the last year honoraria or consulting fees from Best Doctors/Teladoc; Orbus Therapeutics; Adastra Pharmaceuticals; Bristol Meyers Squibb and research funding (instutional contracts) from Orbus; Merck; DNATrix; Abbvie; Beigene; Forma Therapeutics; GCAR; Array BioPharma; Karyopharm Therapeutics; Nuvation Bio; Bayer; Bristol Meyer Squib. W.W. reports to be inventor and patent-holder on “Peptides for use in treating or diagnosing IDH1R132H positive cancers” (EP2800580B1) and “Cancer therapy with an oncolytic virus combined with a checkpoint inhibitor” (US11027013B2). He consulted for Apogenix; Astra Zeneca; Bayer; Enterome; Medac; MSD; and Roche/Genentech with honoraria paid to the Medical Faculty at the University of Heidelberg. MW has received research grants from Apogenix and Quercis, and honoraria for lectures or advisory board participation or consulting from Bayer; Medac; Merck (EMD); Nerviano Medical Sciences; Novartis; Orbus; Philogen and y-Mabs. MAV has patent royalty rights from Infuseon Therapeutics. He has received honoraria from Olympus and Chimerix. His institution has received grant funding from Infuseon Therapeutics; Oncosynergy; and Denovo Pharma for clinical trials for which he is the site primary investigator. EG has received honoraria for advisory board participation from Kiyatec, Inc. (personal compensation) and Karyopharm Therapetuics, Inc. for Data Safety and Monitoring Board participation (compensation to employer). Her institution has received grant funding from Servier Pharmaceuticals LLC (formerly Agios Pharmaceuticals, Inc.), Celgene, MedImmune, Inc. and Tracon Pharmaceuticals.
Figures


Comment in
-
Using historical objective response rates to design single-arm phase II trials in patients with recurrent glioblastoma.Neuro Oncol. 2023 Jun 2;25(6):1029-1030. doi: 10.1093/neuonc/noad046. Neuro Oncol. 2023. PMID: 36881776 Free PMC article. No abstract available.
-
Reply to Di Nunno et al. concerning objective response rates targets for recurrent glioblastoma clinical trials: Toward surrogate endpoints for phase II trials in patients with recurrent glioblastoma.Neuro Oncol. 2023 Aug 3;25(8):1548-1549. doi: 10.1093/neuonc/noad085. Neuro Oncol. 2023. PMID: 37167013 Free PMC article. Clinical Trial. No abstract available.
-
Letter concerning "Objective response rate targets for recurrent glioblastoma clinical trials based on the historic association between ORR and median overall survival": Toward surrogate endpoints for phase II trials in patients with recurrent glioblastoma.Neuro Oncol. 2023 Aug 3;25(8):1546-1547. doi: 10.1093/neuonc/noad071. Neuro Oncol. 2023. PMID: 37171969 Free PMC article. No abstract available.
Similar articles
-
A multicenter phase II study of temozolomide plus disulfiram and copper for recurrent temozolomide-resistant glioblastoma.J Neurooncol. 2019 May;142(3):537-544. doi: 10.1007/s11060-019-03125-y. Epub 2019 Feb 15. J Neurooncol. 2019. PMID: 30771200 Clinical Trial.
-
Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma.J Neurooncol. 2018 Jan;136(1):115-125. doi: 10.1007/s11060-017-2629-z. Epub 2017 Oct 7. J Neurooncol. 2018. PMID: 28988341 Clinical Trial.
-
Procarbazine and CCNU Chemotherapy for Recurrent Glioblastoma with MGMT Promoter Methylation.J Korean Med Sci. 2018 May 10;33(24):e167. doi: 10.3346/jkms.2018.33.e167. eCollection 2018 Jun 11. J Korean Med Sci. 2018. PMID: 29892208 Free PMC article. Clinical Trial.
-
Procarbazine, lomustine and vincristine for recurrent high-grade glioma.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD011773. doi: 10.1002/14651858.CD011773.pub2. Cochrane Database Syst Rev. 2017. PMID: 28744879 Free PMC article. Review.
-
The Role of a Single Angiogenesis Inhibitor in the Treatment of Recurrent Glioblastoma Multiforme: A Meta-Analysis and Systematic Review.PLoS One. 2016 Mar 23;11(3):e0152170. doi: 10.1371/journal.pone.0152170. eCollection 2016. PLoS One. 2016. PMID: 27007828 Free PMC article. Review.
Cited by
-
Systemic inflammatory markers and volume of enhancing tissue on post-contrast T1w MRI images in differentiating true tumor progression from pseudoprogression in high-grade glioma.Clin Transl Radiat Oncol. 2024 Aug 30;49:100849. doi: 10.1016/j.ctro.2024.100849. eCollection 2024 Nov. Clin Transl Radiat Oncol. 2024. PMID: 39318678 Free PMC article.
-
Standardized brain tumor imaging protocols for clinical trials: current recommendations and tips for integration.Front Radiol. 2023 Dec 13;3:1267615. doi: 10.3389/fradi.2023.1267615. eCollection 2023. Front Radiol. 2023. PMID: 38152383 Free PMC article. Review.
-
The Use of Targeted Cytokines as Cancer Therapeutics in Glioblastoma.Cancers (Basel). 2023 Jul 23;15(14):3739. doi: 10.3390/cancers15143739. Cancers (Basel). 2023. PMID: 37509400 Free PMC article. Review.
-
Reply to Di Nunno et al. concerning objective response rates targets for recurrent glioblastoma clinical trials: Toward surrogate endpoints for phase II trials in patients with recurrent glioblastoma.Neuro Oncol. 2023 Aug 3;25(8):1548-1549. doi: 10.1093/neuonc/noad085. Neuro Oncol. 2023. PMID: 37167013 Free PMC article. Clinical Trial. No abstract available.
-
Dendritic cell vaccine trials in gliomas: Untangling the lines.Neuro Oncol. 2023 Oct 3;25(10):1752-1762. doi: 10.1093/neuonc/noad088. Neuro Oncol. 2023. PMID: 37289203 Free PMC article. Review.
References
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Oncology Center for Excellence, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics: Guidence for Industry; 2018. Silver Spring, MD; U.S. DHHS FDA. https://www.fda.gov/media/71195/download. Accessed November 1, 2022.
-
- Axelson M, Liu K, Jiang X, et al. . U.S. food and drug administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013; 19(9):2289–2293. - PubMed
-
- Federman N, Brien EW, Narasimhan V, et al. . Giant cell tumor of bone in childhood: clinical aspects and novel therapeutic targets. Paediatr Drugs. 2014; 16(1):21–28. - PubMed
-
- Wood L. Sunitinib malate for the treatment of renal cell carcinoma. Expert Opin Pharmacother. 2012; 13(9):1323–1336. - PubMed

